Surface modified coated lithium-rich material, preparation method thereof and lithium ion battery
A technology of lithium-rich materials and surface modification, which is applied in the direction of battery electrodes, secondary batteries, and active material electrodes, can solve the problems that lithium-rich materials cannot be solved at the same time, achieve improved first-time efficiency, good reproducibility of results, and preparation The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
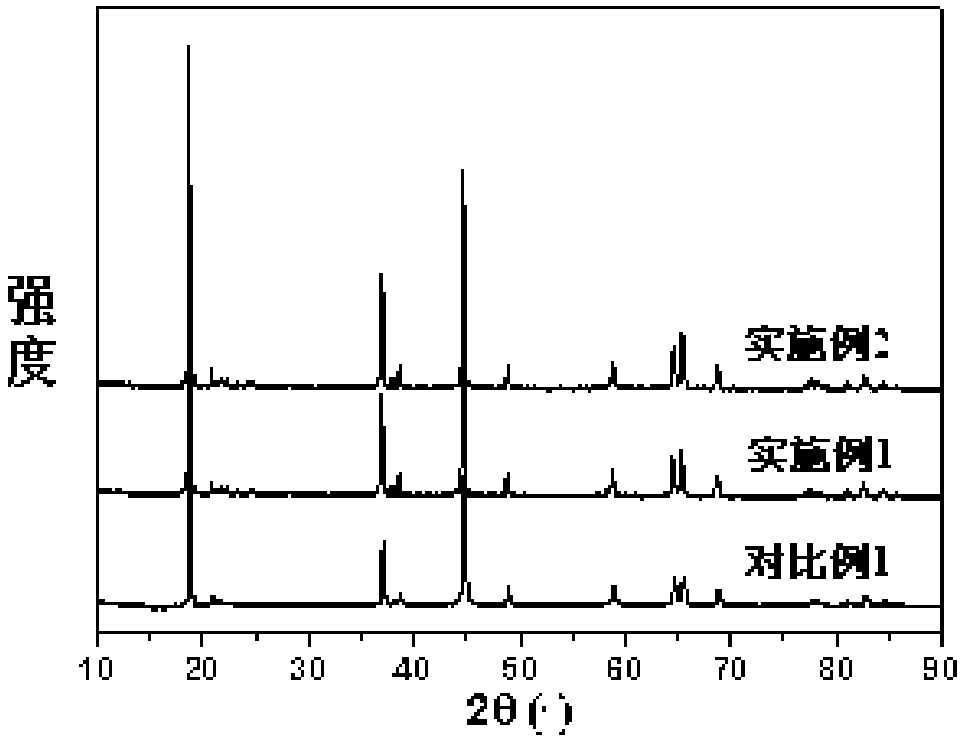
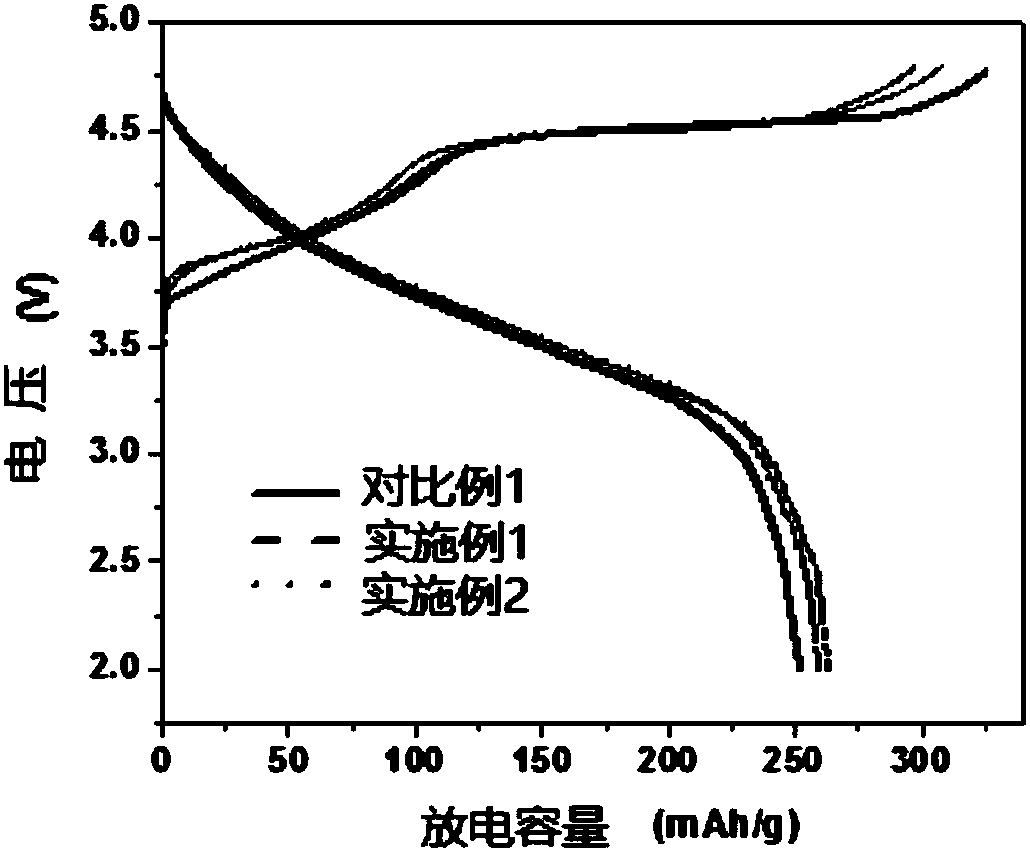
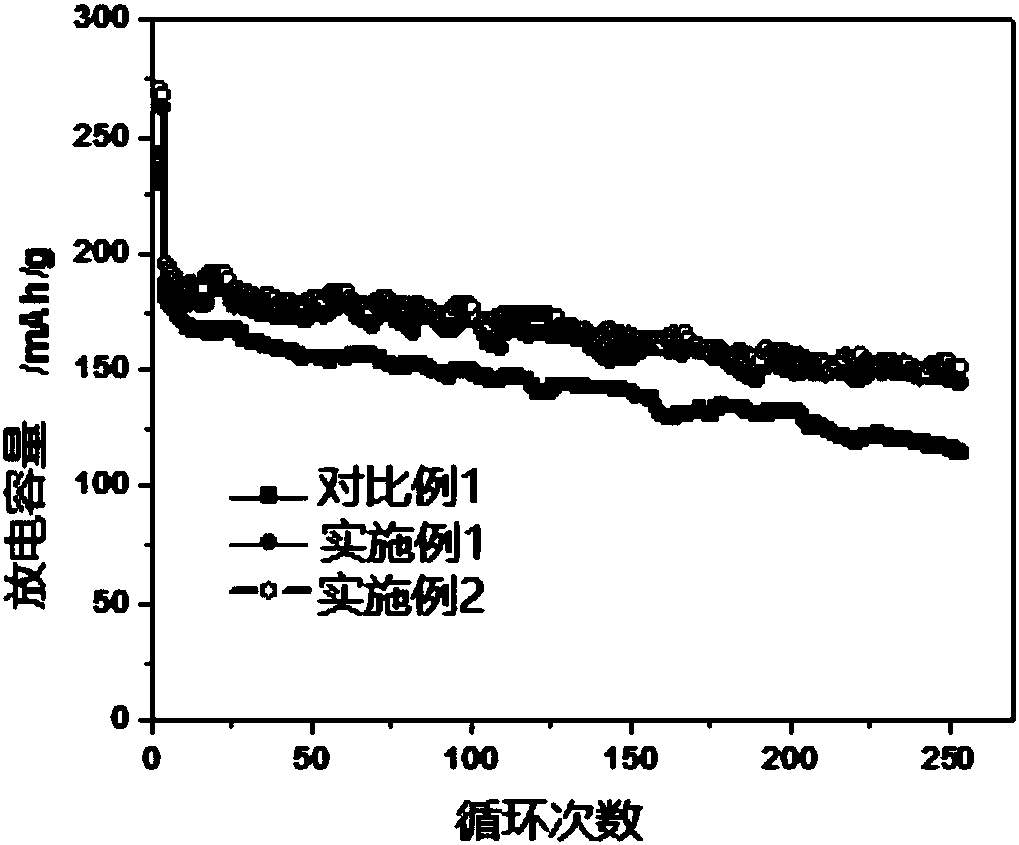
Image
Examples
Embodiment 1
[0057] Surface-modified coated lithium-rich material Li 1.2 mn 0.58 Ni 0.13 co 0.13 S 0.03 o 2.2 Preparation of:
[0058] 1) Prepare lithium-rich material Li with the method of comparative example 1 1.2 mn 0.54 Ni 0.13 co 0.13 o 2
[0059] 2) Preparation of coating layer
[0060] Add the lithium-rich material to 0.2mol / L manganese acetate solution, and keep stirring in a 50°C water bath until dry; the molar ratio of manganese acetate to lithium-rich material is 0.04:1. The dried material was sintered at 600° C. for 5 hours to obtain a lithium-rich material coated with manganese oxide.
[0061] 3) Solution processing
[0062] Add 0.1 mol / L ammonium sulfate solution to the manganese oxide-coated lithium-rich material obtained in step (2), and keep stirring in a 60°C water bath until dry; the molar ratio of ammonium sulfate to lithium-rich material is 0.03:1. The dried material was sintered at 400°C for 2 hours to obtain the ammonium sulfate-modified coated lithium-...
Embodiment 2
[0064] Surface-modified coated lithium-rich material Li 1.2 mn 0.64 Ni 0.13 co 0.13 P 0.05 o 2.3 Preparation of:
[0065] 1) Prepare lithium-rich material Li with the method of comparative example 1 1.2 mn 0.54 Ni 0.13 co 0.13 o 2
[0066] 2) Preparation of coating layer
[0067] Same as step 2 in Example 1, the molar ratio of manganese acetate to lithium-rich material was changed to 0.05:1.
[0068] 3) Solution processing
[0069] Add 0.1 mol / L ammonium phosphate solution to the manganese oxide-coated lithium-rich material obtained in step (2), and keep stirring in a 60°C water bath until dry; the molar ratio of ammonium phosphate to lithium-rich material is 0.05:1. The dried material was sintered at 500°C for 4 hours to obtain the ammonium phosphate-modified coated lithium-rich material Li 1.2 mn 0.64 Ni 0.13 co 0.13 P 0.05 o 2.3 .
Embodiment 3
[0071] Surface-modified coated lithium-rich material Li 1.23 mn 0.57 Ni 0.2 al 0.16 B 0.02 o 2.27 Preparation of:
[0072] 1) Prepare lithium-rich material Li with the method of comparative example 1 1.23 mn 0.57 Ni 0.2 o 2
[0073] 2) Preparation of coating layer
[0074] Same as step 2 in Example 1, the molar ratio of aluminum nitrate to lithium-rich material was changed to 0.16:1.
[0075] 3) Solution processing
[0076] Add 0.05 mol / L boric acid solution to the alumina-coated lithium-rich material obtained in step (2), and keep stirring in a 70°C water bath until dry; the molar ratio of boric acid to lithium-rich material is 0.02:1. The dried material was sintered at 600°C for 7 hours to obtain the boric acid-modified coated lithium-rich material Li 1.23 mn 0.57 Ni 0.2 al 0.16 B 0.02 o 2.27 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com