Application of isorhynchophylline to preparation of medicine with nerve protection effect
A technology of neuroprotection and isorynchophylline, applied in the field of natural medicinal chemistry, can solve problems such as reducing MAO-A activity, achieve good protective effects, expand blood vessels, and slow heart rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This example verifies the neuroprotective effect of isorhynchophylline through the following experimental steps
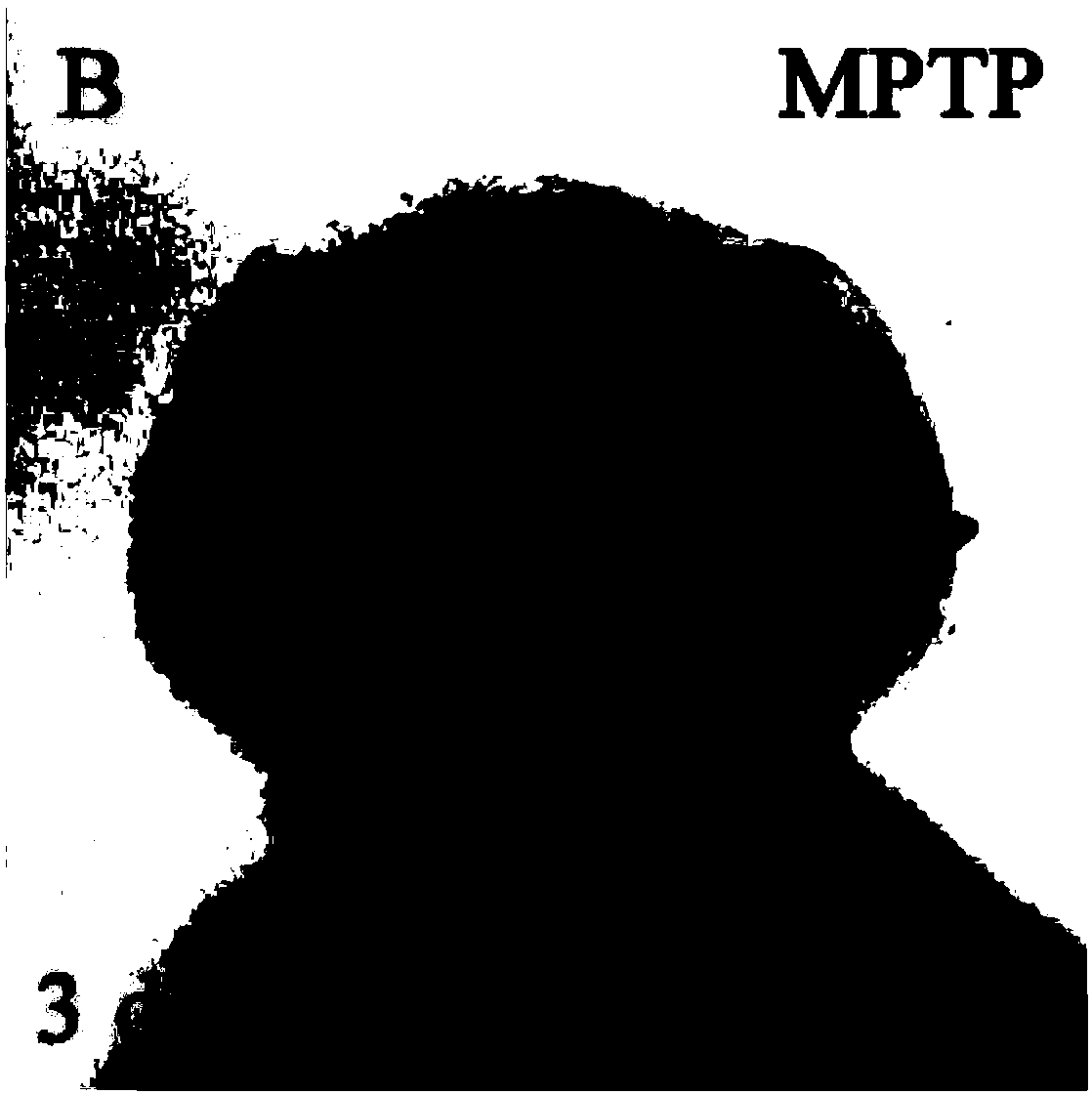
[0031] Dopaminergic neuron-specific toxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) 40 μg / ml treated zebrafish embryos 1-3 days after fertilization, 20 μg / mL Benzylmethynylamine (Deprenyl) was used as a positive control, 20 μM isorhynchophylline (indicated by Z2) was added, and the dopaminergic neuron specific marker gene dat was used as a probe for in situ hybridization to determine the results.
[0032] The result is as figure 1 (control group), figure 2 (MPTP group), image 3 (benzylmethynylamine group and MPTP mixed group) and Figure 4 (mixed group of isorhynchophylline Z2 and MPTP). From the comparison of the figures, it can be concluded that Z2 can effectively reverse the death of dopaminergic neurons induced by MPTP. It is generally believed that Parkinson's disease is caused by the death of dopaminergic neurons, and dat is the marker...
Embodiment 2
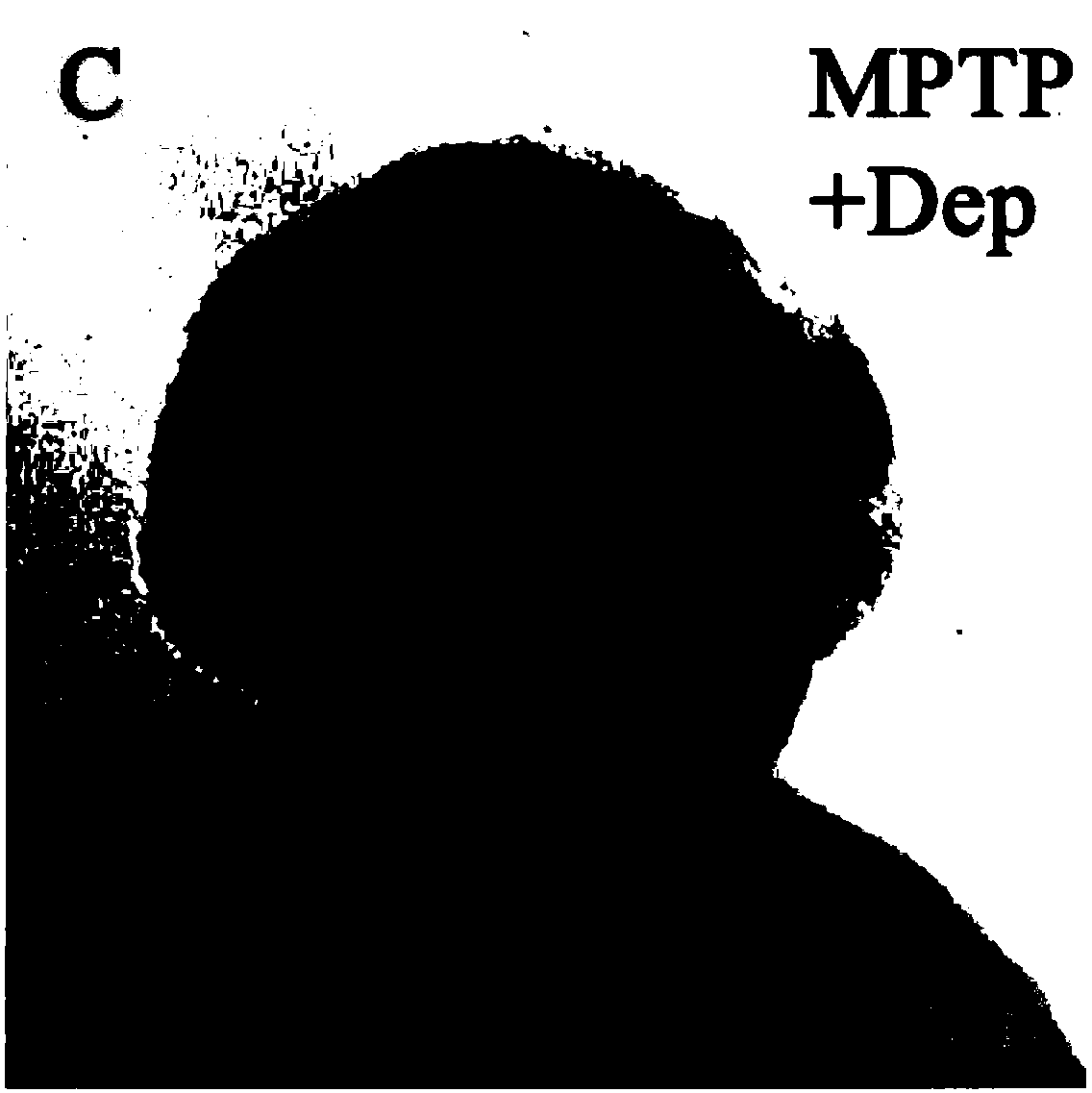
[0034] In this example, the following experiments were performed to detect dopaminergic neurons by in situ hybridization with dat probes, and to observe the changes in the number of dopaminergic neurons before and after drug administration in zebrafish embryos 3 days after fertilization.
[0035] A, adding 0.1% PTU to the embryos of the control group to remove the pigment;
[0036] B, MPTP treatment of embryos for 48 hours;
[0037] C, adding the positive control drug Deprenyl and MPTP to co-treat the embryo for 48 hours;
[0038] D. Embryos treated with MPTP and Z2.
[0039] From the experiment, the normal expression of diencephalic dopaminergic neurons in group A; the death of a large number of diencephalic dopaminergic neurons in group B; the increase of dopaminergic neurons in group C compared with group B; Both elements have recovered significantly, and the protection effect of Z2 is better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com