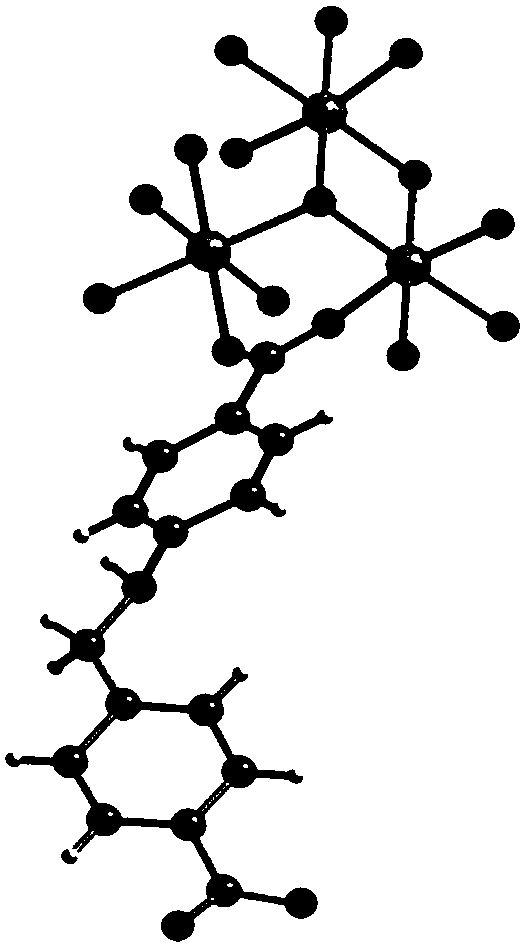
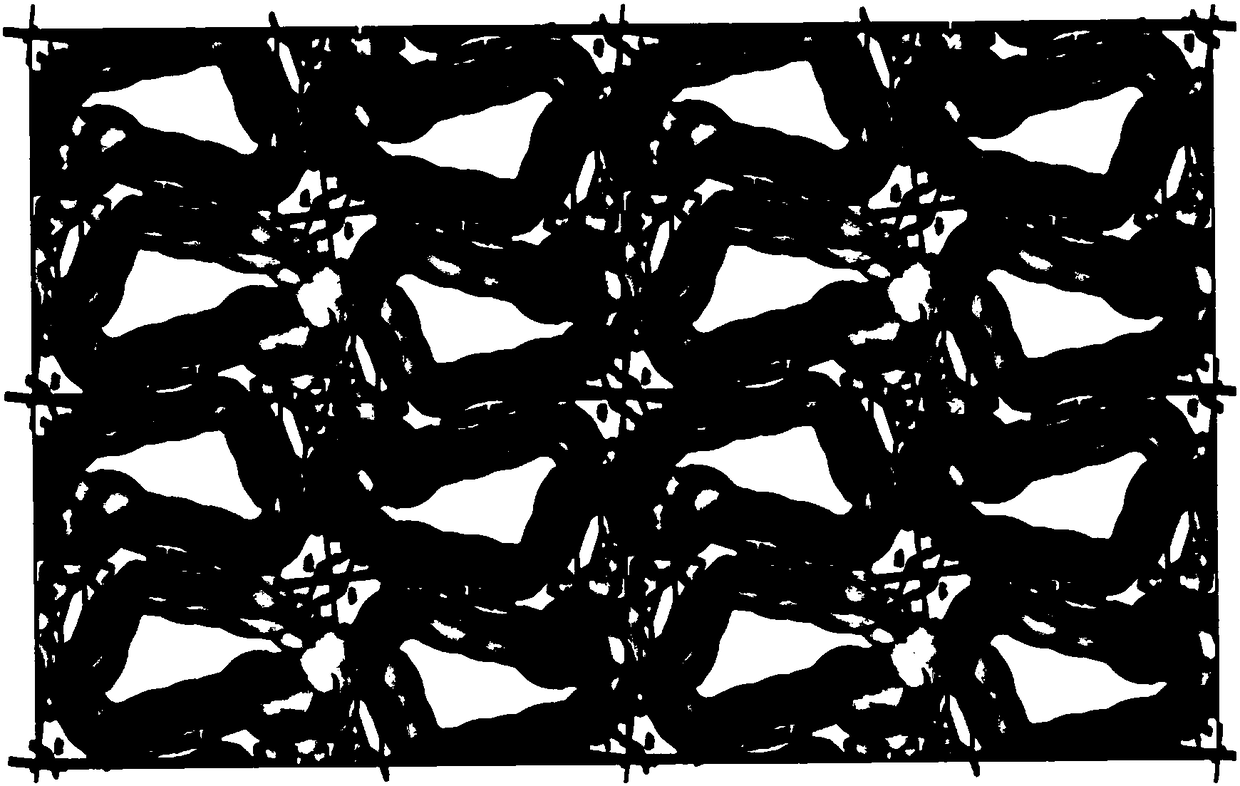
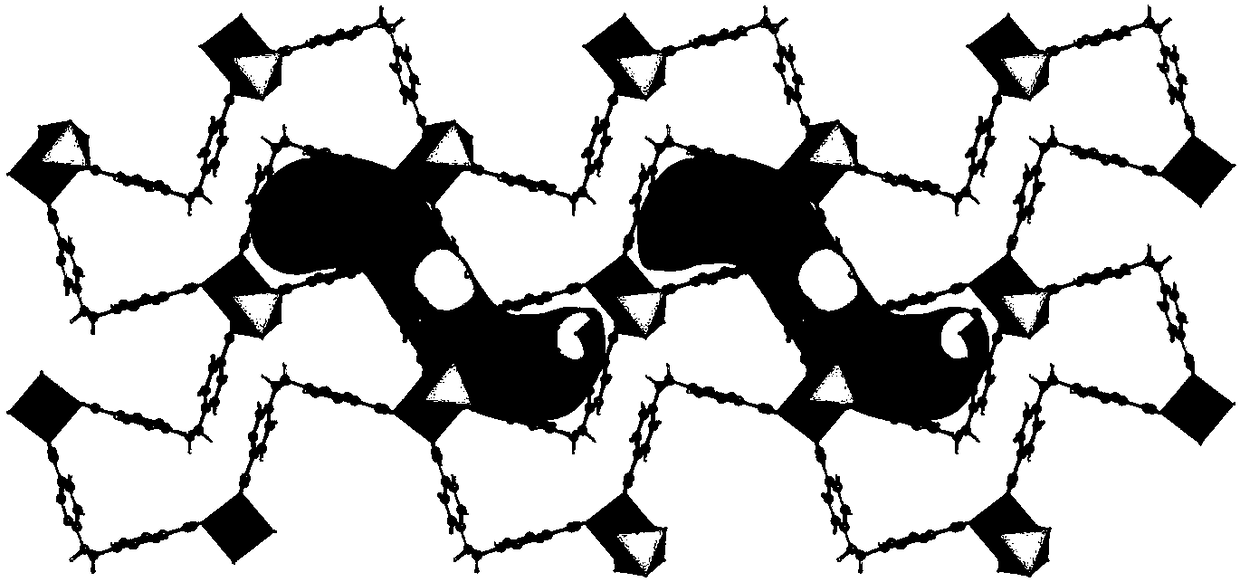
Multifunctional integral catalyst, synthesis method and application
A synthetic method and an integrated technology, applied in catalytic reactions, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of limited catalytic activity, single metals and their oxides do not have catalytic activity, and cannot be applied. Achieve the effects of simple method, easy mass production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The embodiment of the present invention also provides a method for synthesizing the above-mentioned multifunctional integrated catalyst, which includes the following steps:
[0036] (1) Synthesis of H 2 CBBA ligands:
[0037] According to the dosage ratio of 9-12mmol: 9-12mmol: 10-15mmol: 100-200mL, take methyl p-aldehyde benzoate, methyl p-aminobenzoate, anhydrous MgSO 4 and CHCl 3 , Stir the reaction at room temperature for at least 20 h.
[0038] Remove MgSO by filtration 4 , the filtrate was rotary evaporated to dryness, NaBH was added 4 , tetrahydrofuran (THF) and ethanol (EtOH), stirred at room temperature for at least 10 h, NaBH 4 , THF, and EtOH are used in a ratio of 10-12mmol: 50-100mL: 20-30mL.
[0039] The reaction was quenched by adding water under ice bath conditions, EtOH and THF were removed by rotary evaporation, and ethyl acetate (EA) / H 2 O extraction, the organic phases were combined and washed with a saturated NaCl solution, and the EA solvent...
Embodiment 1
[0054] This embodiment provides a multifunctional integrated catalyst CBBA-Co, which is synthesized according to the following method:
[0055]In a 250mL single-necked round bottom flask, add 1.64g (10 mmol) of methyl p-aldehyde benzoate, 1.51 g (10 mmol) of methyl p-aminobenzoate, anhydrous MgSO 4 1.5 g (12.5 mmol) and CHCl 3 150mL, stirred at room temperature for 24h.
[0056] Remove MgSO by filtration 4 , the filtrate was evaporated to dryness with a rotary evaporator, and NaBH was added 4 0.42g (11mmol), THF (75mL) and EtOH (25mL), the reaction was stirred overnight at room temperature.
[0057] Add water to quench the reaction under ice bath conditions, remove EtOH and THF by rotary evaporation, and use EA / H 2 O extraction, combined filtrates and washed with saturated NaCl solution, rotary evaporation to dryness to remove the EA solvent, to obtain a relatively pure esterified ligand.
[0058] Dissolve the esterified ligand with NaOH 3g (75mmol) in H 2 In O / EtOH / T...
Embodiment 2
[0061] This embodiment provides a multifunctional integrated catalyst CBBA-Co, which is synthesized according to the following method:
[0062] In a 250mL single-necked round bottom flask, add 1.48g (9 mmol) of methyl p-aldehyde benzoate, 1.36 g (9 mmol) of methyl p-aminobenzoate, anhydrous MgSO 4 1.2 g (10 mmol) and CHCl 3 (100 mL), the reaction was stirred at room temperature for 24 h.
[0063] Remove MgSO by filtration 4 , the filtrate was evaporated to dryness with a rotary evaporator, and NaBH was added 4 0.38g (10mmol), THF (50mL) and EtOH (20mL), stirred the reaction overnight at room temperature.
[0064] Add water to quench the reaction under ice bath conditions, remove EtOH and THF by rotary evaporation, and use EA / H 2 O extraction, combined organic phases and washed with saturated NaCl solution, rotary evaporation to dryness to remove EA solvent, to obtain a relatively pure esterified ligand.
[0065] Dissolve the esterified ligand and NaOH 2g (50mmol) in H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com