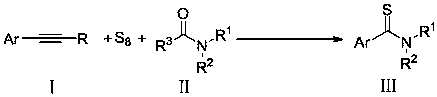
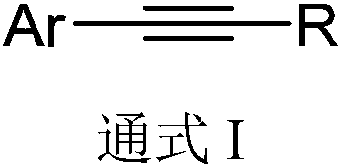
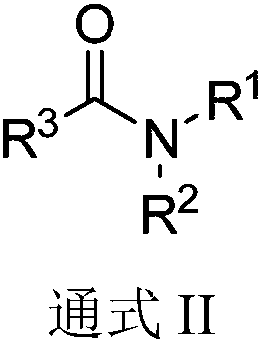
Preparation method of aryl thioamides
A technology of aryl thioamides and amide solvents, which is applied in the field of preparation of aryl thioamide compounds, can solve the problems of low yield, complex structure of reactants, harsh reaction conditions, etc., and achieve simple process and applicable scope of substrates wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Base screening test: Different bases promote the three-component reaction involving aromatic alkynes, resulting in different yields.
[0025] Take phenylacetylene (0.4mmol), elemental sulfur (9.6mmol), alkali (1.2mmol), add 2mL N, N-dimethylformamide to make a mixture, place the mixture in a 10mL pressure-resistant tube and seal it, at 115°C After heating in an oil bath for 20 h, cool to room temperature and add 25 mL of water to quench, the aqueous phase was extracted with dichloromethane (10 mL x 3), the organic phases were combined, and anhydrous MgSO 4 After drying and concentration, add 30 mL of methanol, filter and concentrate, and then separate by thin-layer chromatography to obtain the target product A.
[0026]
[0027] Different bases promote the three-component reaction involving aromatic alkynes, and different yields are obtained. The experimental results are as follows:
[0028]
Embodiment 2
[0030] The preparation method of the aryl thioamide compound A of following structure:
[0031]
[0032] Take phenylacetylene (0.4mmol), elemental sulfur (9.6mmol), anhydrous potassium phosphate (1.2mmol), add 2mL N,N-dimethylformamide to make a mixture, and place the mixture in a 10mL pressure-resistant tube Sealed, heated in an oil bath at 115°C for 20h, cooled to room temperature and quenched with 25mL of water, the aqueous phase was extracted with dichloromethane (10mL x 3), the organic phase was combined, anhydrous MgSO 4 After drying and concentration, add 30 mL of methanol, filter and concentrate, and then separate by thin-layer chromatography to obtain 56 mg of the target product A as a yellow solid, with a yield of 84%. NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ7.31–7.28(m,5H),3.55(s,3H),3.11(s,3H); 13 C NMR (CDCl 3 , 100MHz) δ200.9, 143.3, 128.6, 128.3, 125.7, 44.2, 43.2.
Embodiment 3
[0034] The only difference between this example and Example 2 is that the solvent used was N,N-dimethylacetamide (2 mL), and 44 mg of the target product A was obtained with a yield of 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com