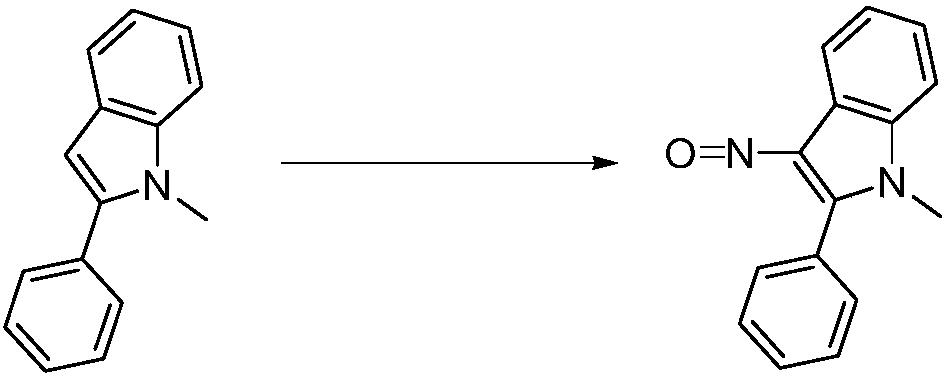
Preparation method of 3-nitroso-2-phenyl-1H-indole
A nitroso and phenyl technology, applied in the field of organic chemical synthesis, can solve problems such as low yield, environmental pollution, and increased reaction risk coefficient, and achieve the effects of high yield, short synthesis process, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
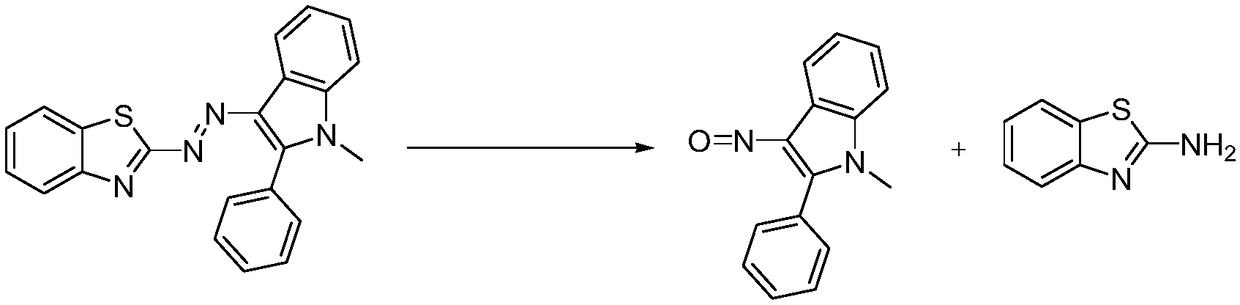
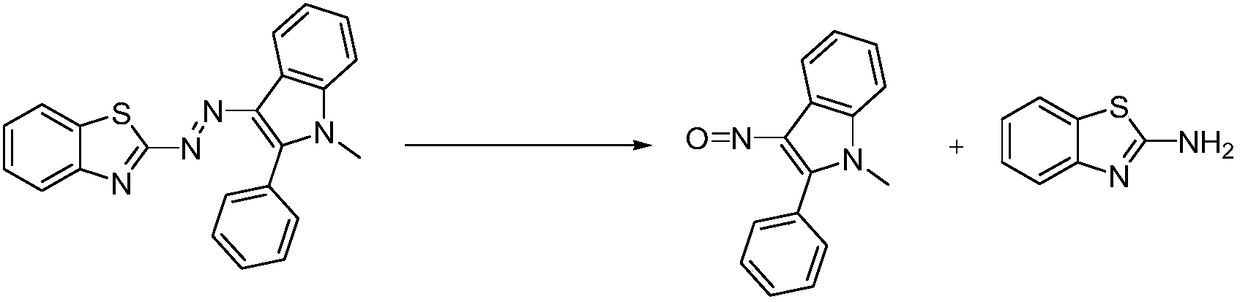
[0026] A preparation method of 3-nitroso-2-phenyl-1H-indole:
[0027] At 20ˉ30°C, tetrahydrofuran (500g) was added to a three-necked flask, and then 2-[2-(1-methyl-2-phenyl-1H-indol-3-yl)azo]benzothiazole ( 50g, 1.0eq) was added in the there-necked flask, stirred to dissolve completely; Potassium hydroxide (15.2g, 2.0eq) was added to 50g of water to prepare aqueous potassium hydroxide solution;
[0028] At 0°C, add potassium hydroxide aqueous solution dropwise to the reaction system in the three-necked flask, and after the dropwise addition, raise the temperature of the system to 50-60°C and react for 8ˉ10h. After the reaction is over, cool down the reaction system to 0-10°C;
[0029] Slowly add 10% dilute hydrochloric acid dropwise to the above reaction system, adjust the pH value of the system to 1-2, separate the organic phase, extract the product with methyl tert-butyl ether (500g) in the water phase, combine the organic phase, add water (250g) and stir to wash Organic p...
Embodiment example 2
[0031] A preparation method of 3-nitroso-2-phenyl-1H-indole:
[0032] At 20ˉ30°C, tetrahydrofuran (500g) was added to a three-necked flask, and then 2-[2-(1-methyl-2-phenyl-1H-indol-3-yl)azo]benzothiazole ( 50g, 1.0eq) was added in the there-necked flask, stirred to dissolve completely; sodium hydroxide (10.9g, 2.0eq) was added to 50g of water to prepare aqueous sodium hydroxide solution;
[0033] At 0°C, add the sodium hydroxide aqueous solution dropwise to the reaction system in the three-necked flask. After the dropwise addition, raise the temperature of the system to 50-60°C and react for 8ˉ10h. After the reaction is over, cool down the reaction system to 0-10°C;
[0034] Slowly add 10% dilute hydrochloric acid dropwise to the above reaction system, adjust the pH value of the system to 1-2, separate the organic phase, extract the product with methyl tert-butyl ether (500g) in the water phase, combine the organic phase, add water (250g) and stir to wash Organic phase, liq...
Embodiment example 3
[0036] At 20ˉ30°C, acetonitrile (500g) was added to a three-necked flask, and then 2-[2-(1-methyl-2-phenyl-1H-indol-3-yl)azo]benzothiazole ( 50g, 1.0eq) was added in the there-necked flask, stirred to dissolve completely; Potassium hydroxide (15.2g, 2.0eq) was added to 50g of water to prepare aqueous potassium hydroxide solution;
[0037] At 0°C, add potassium hydroxide aqueous solution dropwise to the reaction system in the three-necked flask, and after the dropwise addition, raise the temperature of the system to 50-60°C and react for 8ˉ10h. After the reaction is over, cool down the reaction system to 0-10°C;
[0038] Slowly add 10% dilute hydrochloric acid dropwise to the above reaction system, adjust the pH value of the system to 1-2, separate the organic phase, extract the product with methyl tert-butyl ether (500g) in the water phase, combine the organic phase, add water (250g) and stir to wash Organic phase, liquid separation, add anhydrous sodium sulfate (100g) to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com