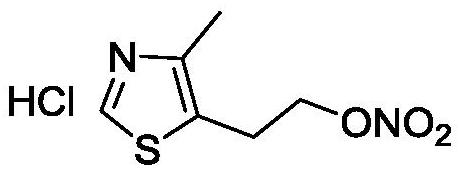
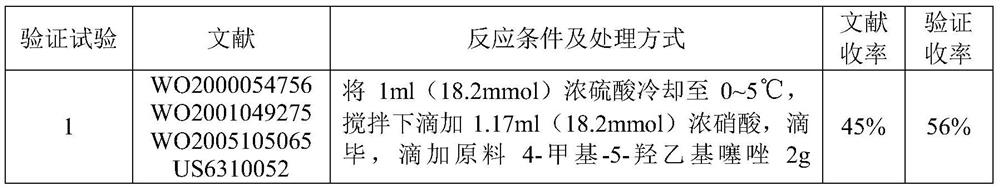
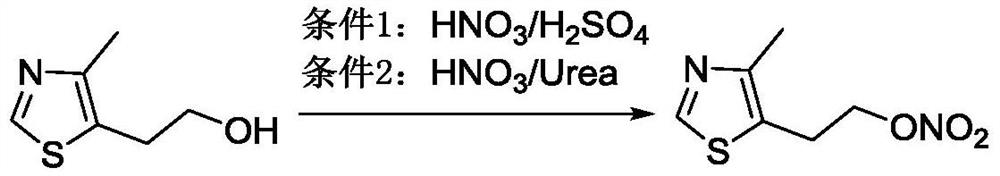The preparation method of 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride
A technology of methylthiazole and hydrochloride, applied in the field of compound preparation, can solve the problems of increased production difficulty and cost, small preparation amount, poor economy and the like, and achieves the advantages of avoiding column chromatography separation and purification, mild reaction conditions, and avoiding explosion risks. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Put 21.46g (0.15mol) of 4-methyl-5-hydroxyethylthiazole in a 1000ml three-necked flask, add 210ml of dichloromethane, cool in an ice-salt bath, and keep the internal temperature below 0°C, add 56.7ml (0.6mol) Acetic anhydride, keep the internal temperature below 0°C, add 25.4ml (0.6mol) fuming nitric acid dropwise, keep 0-4°C for 2 hours after the addition, and the reaction is complete. Add 100ml of water to the solution, keep the internal temperature below 0°C, add dropwise 60% NaOH (about 120ml) aqueous solution to adjust pH≈7~8, separate the water layer, extract with dichloromethane (100ml×2 times), and combine the organic layers , washed with water (100ml × 2 times), washed with saturated brine (100ml × 1 time), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 26.33g of 2-(4-methylthiazol-5-yl)ethyl nitrate, Yield: 93%.
Embodiment 2
[0046]Put 7.15g (0.05mol) of 4-methyl-5-hydroxyethylthiazole in a 500ml three-neck flask, add 70ml of dichloromethane, cool in an ice-salt bath, and keep the internal temperature below 5°C, add 7.3ml (0.08mol) Acetic anhydride, keep the internal temperature below 5°C, add 8.5ml (0.2mol) fuming nitric acid dropwise, keep the internal temperature below 5°C after the addition and react for 2 hours, the reaction is complete, add 33ml of water to the solution, maintain the internal temperature at 5°C Next, add 60% NaOH aqueous solution dropwise to adjust the pH to ≈7~8, separate the aqueous layer, extract with dichloromethane (30ml×2 times), combine the organic layers, wash with water (30ml×2 times), and wash with saturated brine (30ml×2 times). 1 time), dried over anhydrous sodium sulfate, added 0.9 g of activated carbon for decolorization, filtered, and concentrated to obtain 9.10 g of 2-(4-methylthiazol-5-yl)ethyl nitrate, yield: 96.8%.
Embodiment 3
[0048] Put 7.15g (0.05mol) of 4-methyl-5-hydroxyethylthiazole in a 500ml three-necked flask, add 35ml of dichloromethane, cool in an ice-salt bath, and keep the internal temperature below 5°C, add 7.6ml (0.08mol) Acetic anhydride, keep the internal temperature below 5°C, add 8.5ml (0.2mol) fuming nitric acid dropwise, keep the internal temperature below 5°C after adding and react for 3 hours, the reaction is complete, keep the internal temperature below 5°C, add 30% NaOH dropwise Adjust the pH of the aqueous solution to ≈7~8 (about 60ml), separate the aqueous layer, extract with dichloromethane (30ml×2 times), combine the organic layers, wash with water (30ml×2 times), and wash with saturated brine (30ml×1 time) , dried over anhydrous sodium sulfate, decolorized by adding 0.9 g of activated carbon, filtered, and concentrated to obtain 8.94 g of 2-(4-methylthiazol-5-yl)ethyl nitrate, yield: 95.1%. Dissolve the obtained 2-(4-methylthiazol-5-yl)ethyl nitrate in 55ml of ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com