Method for separating flavonoid compounds from ginkgo biloba extract by utilizing simulated moving bed
A technology that simulates moving bed and flavonoids, which can be used in drug combination, organic chemistry, allergic diseases, etc., and can solve problems such as research on limiting biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
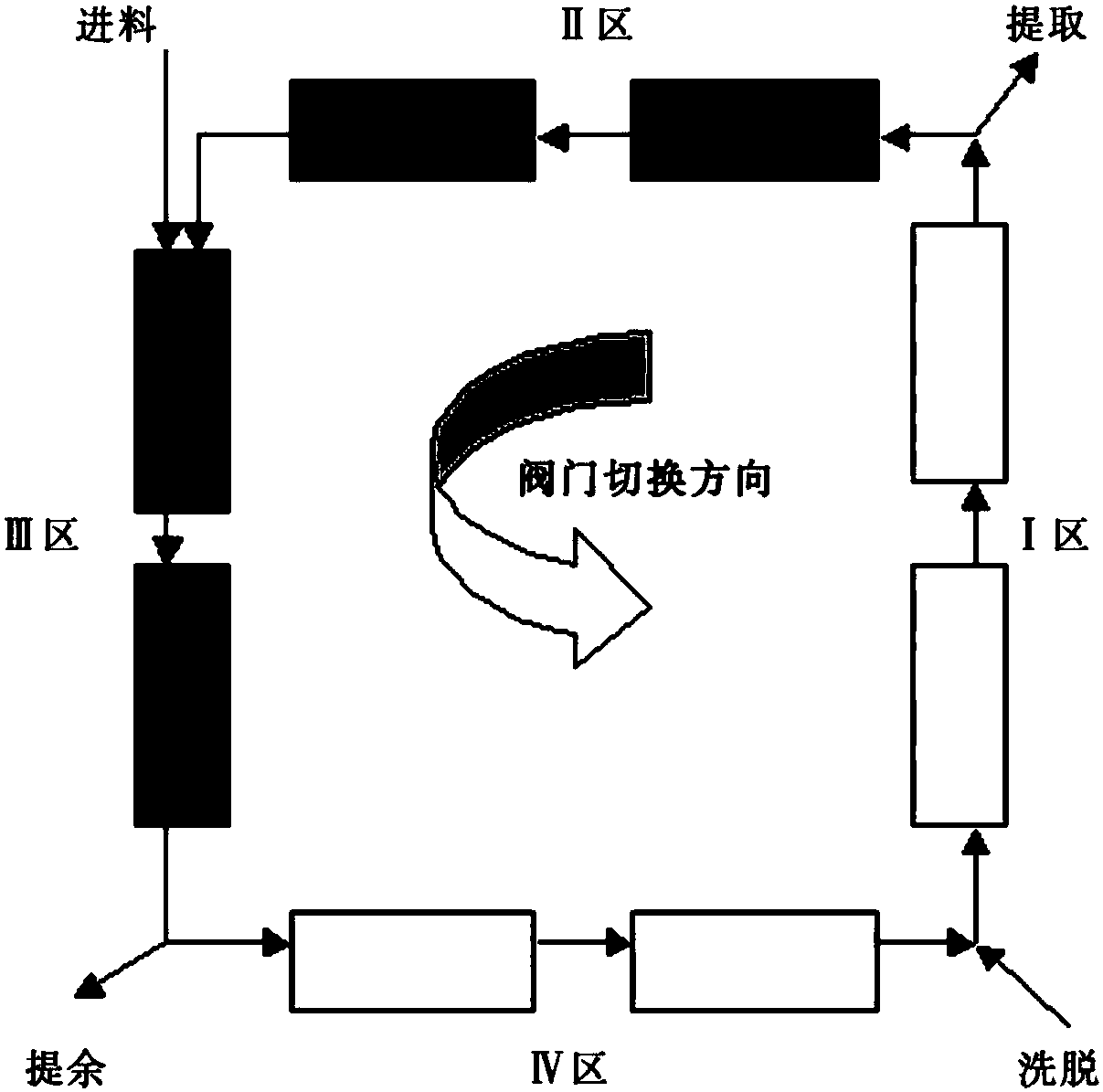
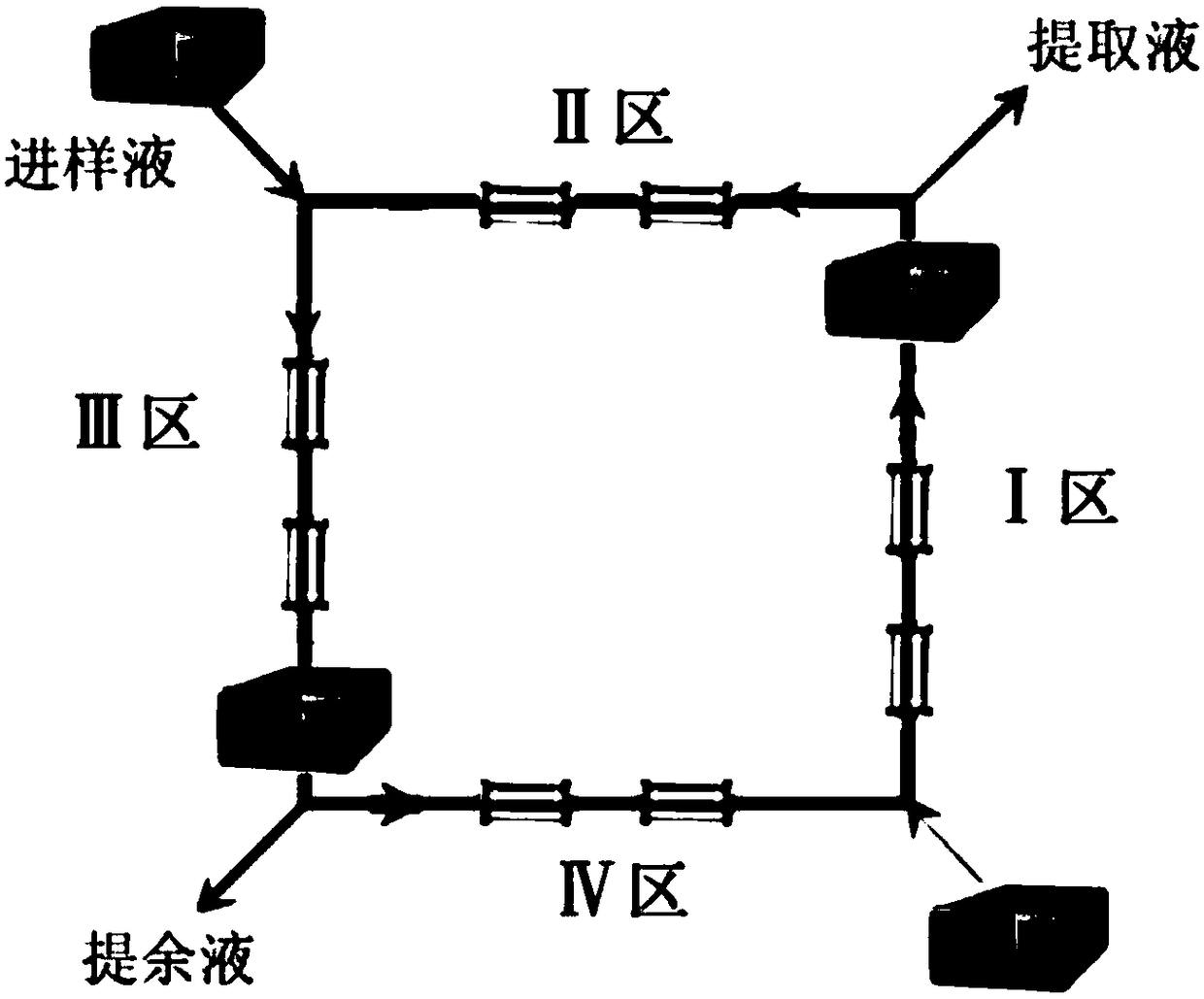
Image
Examples
Embodiment 1
[0032] (1) Take dried ginkgo leaves (1.0kg) and crush them to 20-80 meshes, add β-glucosidase aqueous solution (2.5L) with a mass fraction of 6% to soak for 8 hours, and then add 1:1 volume ratio of Hydroethanol and 3% Na 2 CO 3 The mixed solution (10L) of the solution was extracted under reflux for 12h, filtered, and the filtrate was concentrated to obtain 11.2g of crude extract;
[0033] (2) Take 10 g of the crude extract obtained in step (1) and disperse it with 150 mL of water, adjust the pH to 4.0-5.0 with dilute hydrochloric acid, extract twice with ethyl acetate, combine the organic phases, and concentrate to obtain 1.80 g of the extract ;
[0034] (3) The extract (1.80g) obtained in step (2) is adsorbed by AB-8 type macroporous resin, and is eluted with 95% ethanol for 3 column volumes with a volume fraction, and the eluent is concentrated and dried to obtain the ginkgo biloba Lute flavone extract 865mg, hereinafter referred to as product A.
Embodiment 2
[0036] (1) Take dried ginkgo leaves (1.0kg) and crush them to 20-80 meshes, add 8% β-glucosidase aqueous solution (2.0L) to soak for 6 hours, then add 1:1 volume ratio of Hydroethanol and 5% Na 2 CO 3 The mixed solution (8L) of the solution was extracted by reflux for 10h, filtered, and the filtrate was concentrated to obtain 11.8g of crude extract;
[0037] (2) Take 10 g of the crude extract obtained in step (1) and disperse it with 100 mL of water, adjust the pH to 4.0-5.0 with acetic acid, extract 3 times with ethyl acetate, combine the organic phases, and concentrate to obtain 1.82 g of the extract;
[0038] (3) The extract (1.82g) obtained in step (2) is adsorbed by D101 type macroporous resin, and 5 column volumes are eluted with ethanol with a volume fraction of 95%, and the eluate is concentrated and dried to obtain the ginkgo leaf flavonoids Extract 881mg, hereinafter referred to as product B.
Embodiment 3
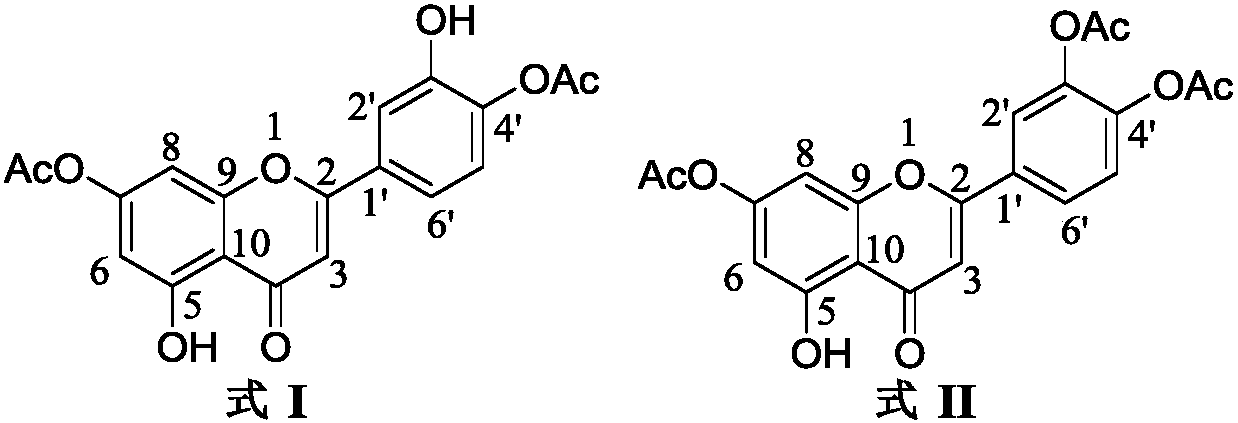
[0040] Get product A (50mg) first through Sephadex LH-20 gel column chromatography, eluent is CH 2 Cl2 : MeOH=1:1, and then prepared by high performance liquid chromatography HPLC, the chromatographic column is Hypersil 300A C18, 10×250mm, 10μm, the flow rate is 2mL / min, and the mobile phase is MeOH:H 2 O=55:45 gave the compound of formula I (7.3 mg) and compound of formula II (5.2 mg), respectively.
[0041]
[0042] The structure confirmation data of the compound of formula I: ESIMS m / z 371.1[M+H] + , 1 H, 13 C NMR data is shown in the table below; structure confirmation data of the compound of formula II: ESIMS m / z 413.1[M+H] + , 1 H, 13 C NMR data are given in the table below.
[0043] Formula I, II 1 H, 13 C NMR data (Acetone-d 6 , 400 / 100MHz)
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com