siRNA capable of inhibiting expression of COL1A1 gene in human beings and animals and composition containing same, and application thereof
A technology of amine compounds and modifiers, applied in DNA/RNA fragments, drug combinations, gene therapy, etc., can solve the problems that hinder the development of siRNA drugs, poor siRNA activity, and slow progress in clinical application, so as to shorten the process of animal experiments, Improved developmental progression, significant mRNA repression effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
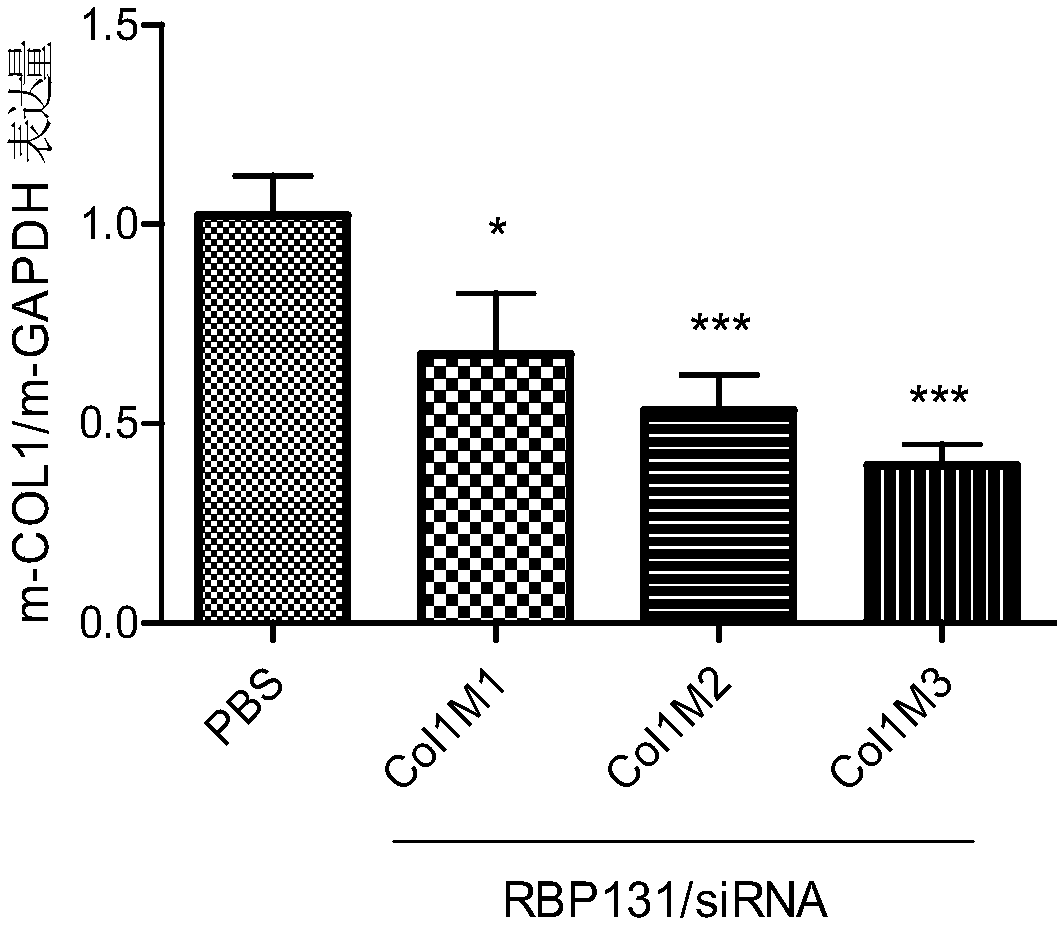
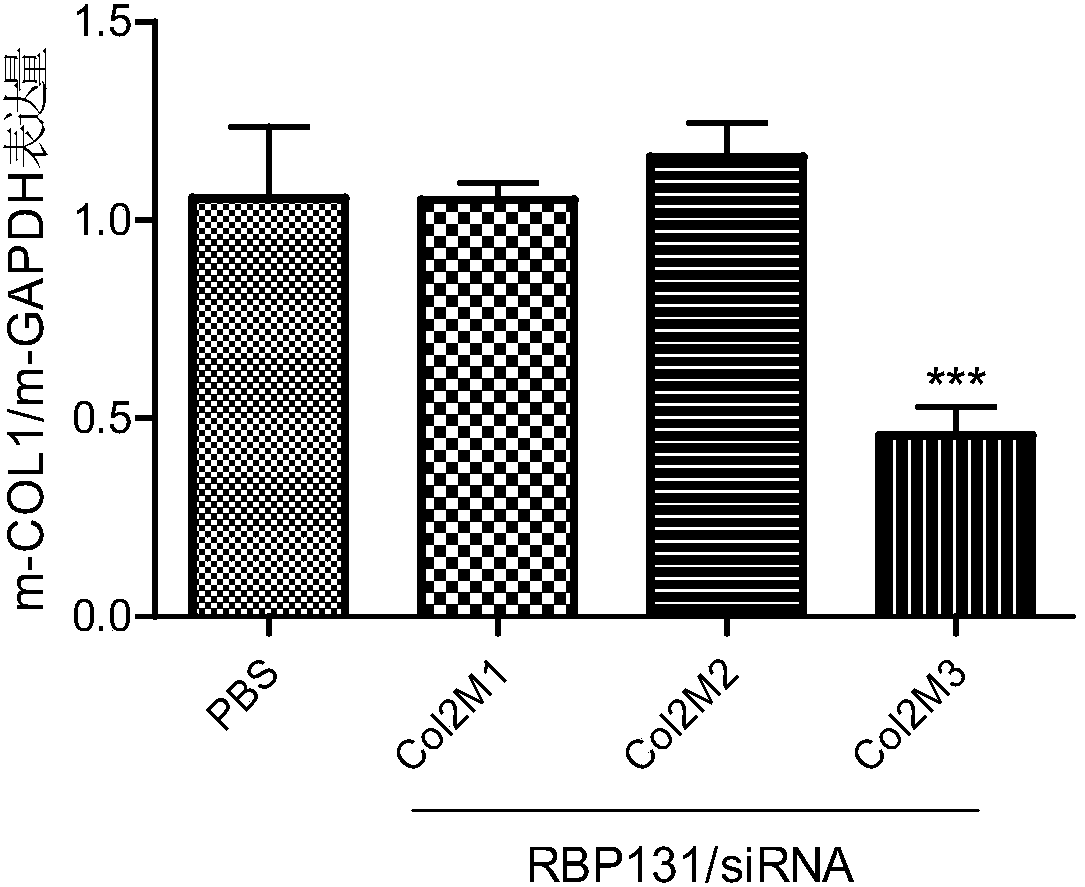
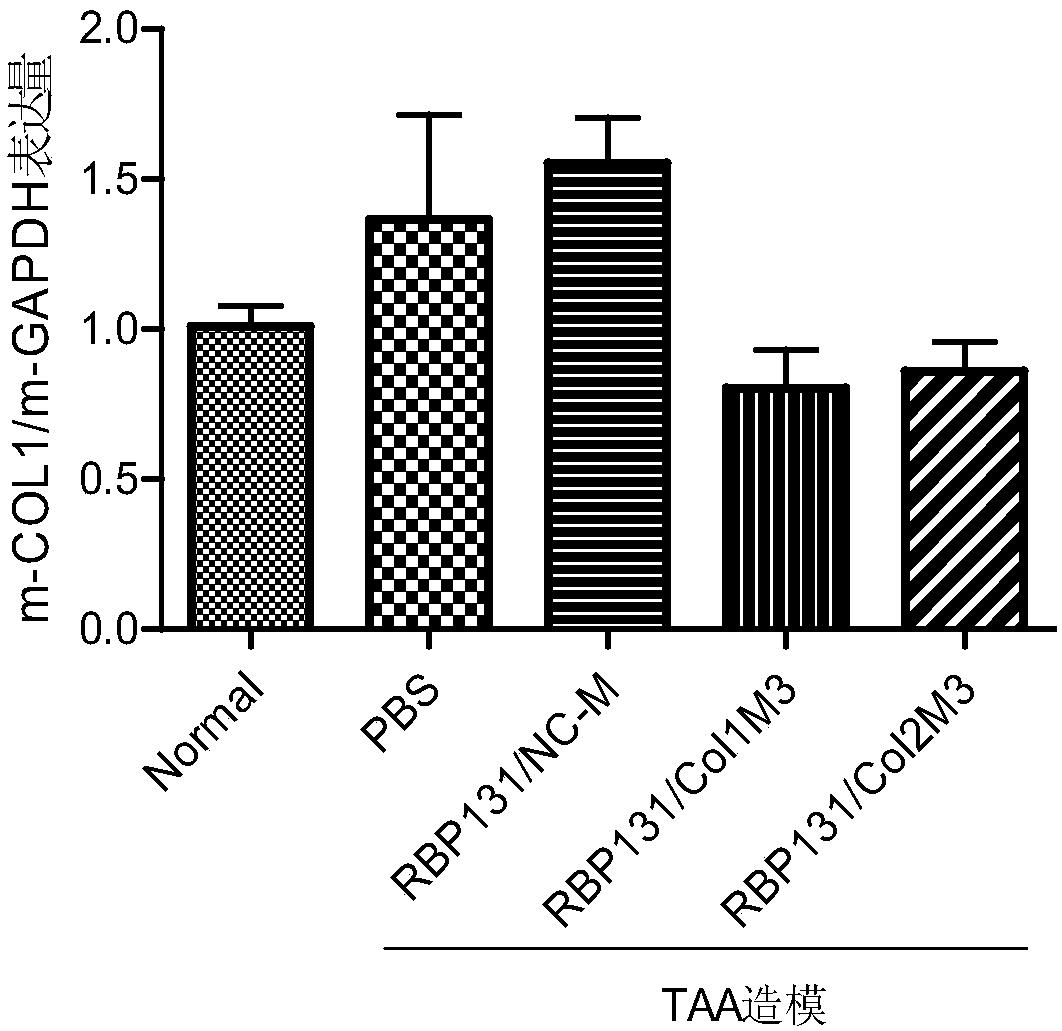
[0120] The present invention uses COL1A1mRNA (Genebank registration number: (NM_000088.3) as a template for siRNA design to obtain 3 species-conserved siRNAs, whose sequence information is shown in Table 1. At the same time, set the sense strand nucleotide sequence such as SEQ ID NO.11 The siRNA whose antisense chain nucleotide sequence is shown as SEQ ID NO.12 is numbered NC. NC is an irrelevant sequence that has no corresponding target action site with COL1A1 mRNA, and serves as a negative control.
[0121] Table 1: 7 siRNAs targeting COL1A1
[0122]
[0123]
[0124] The above siRNAs have a high degree of homology among different species, specifically, siRNA Col1 completely matches the target sequence (GAAUGGAGAUGAUGGGGAA, SEQ ID No.21) of human (NM_000088.3) and mouse (NM_007742.4); There is one nucleotide mismatch in the target sequence (GAACGGAGAUGAUGGGGAA, SEQID No.22) of mouse (NM_053304.1) and rhesus monkey (XM_015119317.1), that is, the 4th nucleotide mismatch....
experiment Embodiment 1
[0127] This experimental example is used to detect the inhibitory rate of the siRNA obtained in Preparation Example 1 on the expression level of COL1A1 mRNA in vitro.
[0128] Human cervical cancer cell line (Hela) (purchased from ATCC, CCL-2 TM ) was inoculated and cultured in a 24-well plate with DMEM complete medium containing 10% fetal bovine serum, 2mM L-glutamine, 100U / ml penicillin, and 100mg / ml streptomycin, and the seeding density was 4×10 5 Cells / well, 0.5ml per well, culture overnight at 37°C.
[0129] The specific operation steps of cell transfection are as follows: Dilute 500ng of each siRNA sample synthesized in Example 1 into 50 μl of Opti-MEM serum-free medium, and simultaneously add 1 μl of Lipofectamine TM 2000 (Invitrogen Company) was diluted in 50 μl of Opti-MEM serum-free medium, and the above two solutions were incubated at room temperature for 5 minutes, and mixed evenly. After the mixed solution was allowed to stand at room temperature for 20 minute...
preparation Embodiment 2
[0142] Reasonable chemical modifications were performed on the siRNA CT with good activity and the negative control NC, and the modification information is shown in Table 4.
[0143] Table 4: Sequences of Modified siRNA Molecules
[0144]
[0145] Among them, the uppercase letters C, G, U, A and T represent the base composition of nucleotides; the lowercase letter d indicates that the nucleotide on the right side of the letter d is a deoxyribonucleotide; the lowercase letter m indicates that the letter m The ribose group of one nucleotide on the left is 2'-methoxyribose group; the lowercase letter f indicates that the ribose group of one nucleotide to the left of the letter f is 2'-fluororibose group; the lowercase letter s indicates that the phosphate group between the deoxyribonucleotides on both sides of the letter is a phosphorothioate group.
[0146]The sense and antisense strands of the siRNAs listed in Table 4 were obtained by conventional solid-phase synthesis meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com