Enrichment method for peripheral blood free tumor DNA, kit and application thereof
A kit and peripheral blood technology, applied in the field of tumor detection, can solve the problems of insufficient capture efficiency, prolonged delivery time, and long capture time, and achieve the effects of improving capture efficiency, shortening capture time, and high capture efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
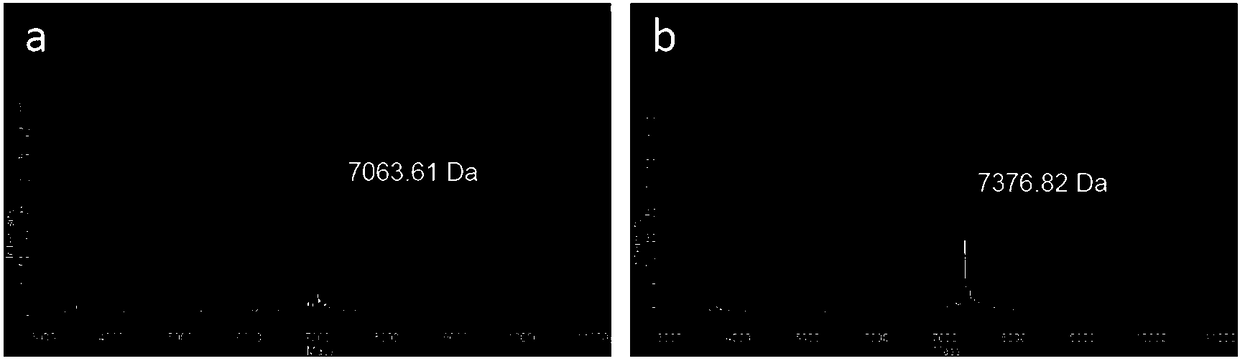
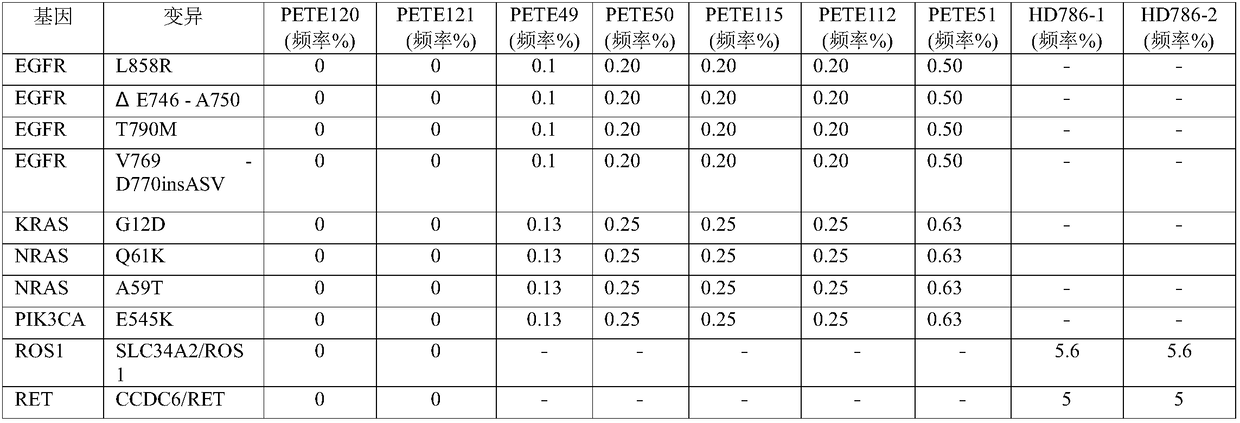
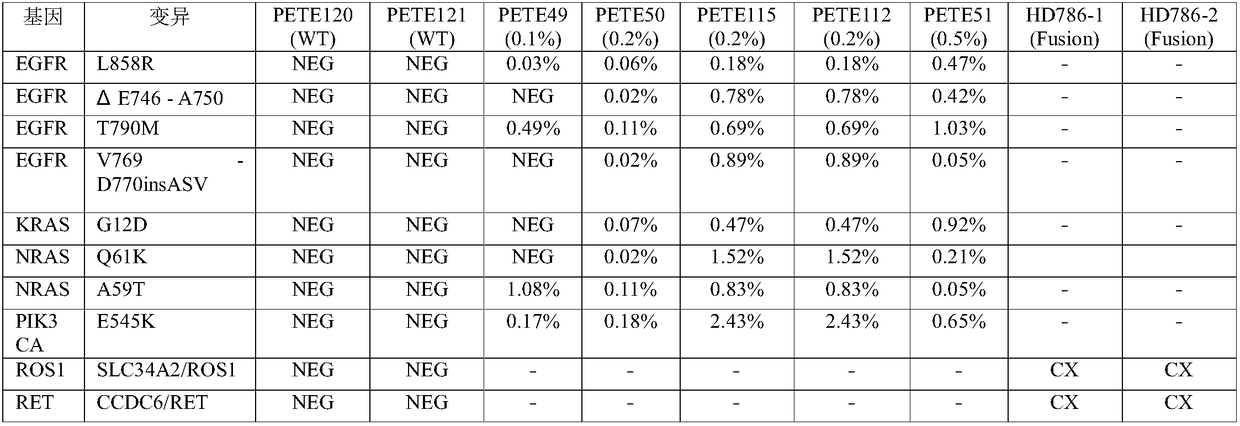
[0055] In this case, three cfDNA reference products covering lung cancer mutations purchased from Horizon were used for the test. The sample numbers of the three cfDNA reference products are HD777, HD779, and HD786; for the specific lung cancer mutation information of the three cfDNA reference products, refer to the official website of Horizon. The URL is as follows:
[0056] https: / / www.horizondiscovery.com / .
[0057] In this example, 1276 PAP probes were used to enrich the lung cancer ctDNA in the cfDNA library, among which 1276 PAP probes covered AKT1, ALK, BRAF, EGFR, ERBB2, FGFR3, KRAS, MAP2K1, MET, NRAS, NTRK1, 15 genes including PIK3CA, RET, ROS1 and TP53. The detailed steps are as follows:
[0058] 1. Preparation of PAP probe
[0059] (1) ddNTP modification
[0060] The PAP probe in this example consists of a universal primer sequence and a specific recognition sequence from the 5' end to the 3' end. The 3' end of the specific recognition sequence includes a 40bp c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com