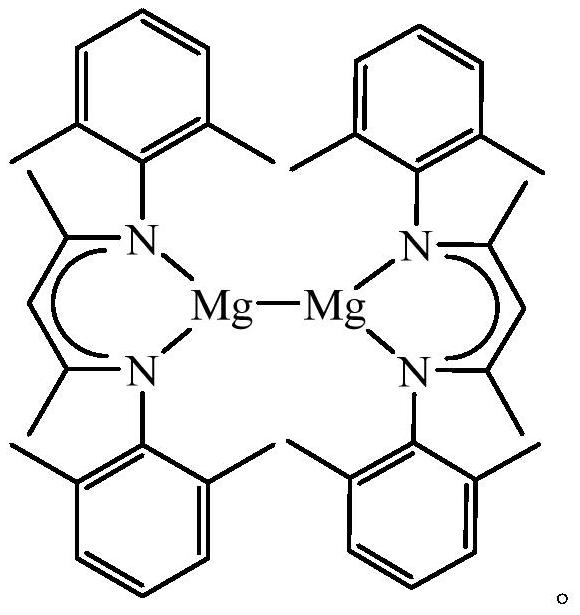
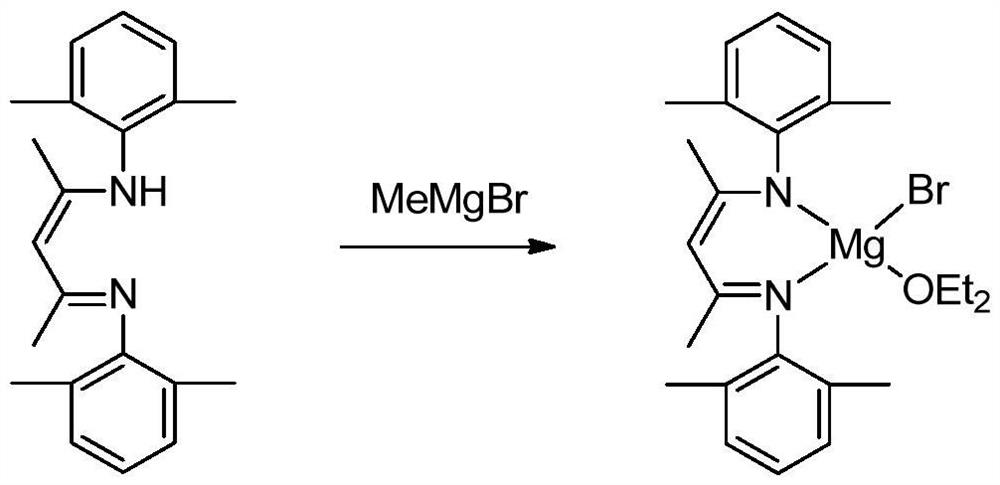
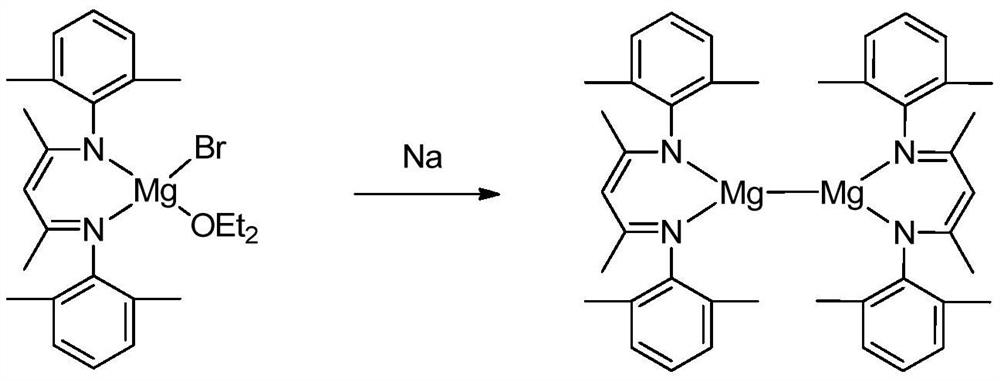
A kind of hydroboration method of ester
A hydroboration and catalyst technology, applied in the field of hydroboration, can solve the problem that the application of the catalytic amount of a monovalent magnesium compound is not reported, and achieve the effects of wide substrate universality, low toxicity and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of the bromide of β-diimine magnesium, the process is as follows:
[0022] In the absence of water and oxygen, 3.27mmol of β-diimine ligand was dissolved in 25mL ether solution in a single-port reaction tube, and 3.92mmol of methylmagnesium bromide was added dropwise to the above solution at -80°C, and reacted at room temperature for 24h. After filtration, the solid was sucked dry, and the filtrate was concentrated to 5 mL to obtain colorless crystals. The mass of solid and crystals was 1.42 g, and the yield was 90%. M.p. 271-273°C. NMR spectrum: 1 HNMR (600MHz, C 6 D. 6 ):δ6.99-6.91(m,6H,Ar-H),4.88(s,1H,CH),3.12(s,4H,OCH 2 CH 3 ),2.65(s,6H,CH 3 ),2.08(s,6H,CH 3 ),1.55(s,6H,NCCH 3 ),0.48(s,6H,OCH 2 CH 3 ) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ):δ168.87 (NCCH 3 ), 147.75, 131.57, 129.56, 124.76 (Ar-C), 95.31 (=CH), 65.96 (OCH 2 CH 3 ),23.52(OCH 2 CH 3 ), 21.09 (NCCH 3 ), 18.89, 13.15 (CH 3 ) ppm.
Embodiment 2
[0024] The preparation of the bromide of β-diimine magnesium, the process is as follows:
[0025] In the absence of water and oxygen, 3.27 mmol of β-diimine ligand was dissolved in 25 mL of ether solution in a single-port reaction tube, and 3.60 mmol of methylmagnesium bromide was added dropwise to the above solution at -60°C, and reacted at room temperature for 15 h. After filtration, the solid was sucked dry, and the filtrate was concentrated to 5 mL to obtain colorless crystals. The mass of solid and crystals was 1.46 g, and the yield was 92%. M.p. 271-273°C. NMR spectrum: 1 HNMR (600MHz, C 6 D. 6 ):δ6.99-6.91(m,6H,Ar-H),4.88(s,1H,CH),3.12(s,4H,OCH 2 CH 3 ),2.65(s,6H,CH 3 ),2.08(s,6H,CH 3 ),1.55(s,6H,NCCH 3 ),0.48(s,6H,OCH 2 CH 3 ) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ):δ168.87 (NCCH 3), 147.75, 131.57, 129.56, 124.76 (Ar-C), 95.31 (=CH), 65.96 (OCH 2 CH 3 ),23.52(OCH 2 CH 3 ), 21.09 (NCCH 3 ), 18.89, 13.15 (CH 3 ) ppm.
Embodiment 3
[0027] The preparation of the bromide of β-diimine magnesium, the process is as follows:
[0028] In the absence of water and oxygen, 3.27 mmol of β-diimine ligand was dissolved in 25 mL of ether solution in a single-port reaction tube, and 3.27 mmol of methylmagnesium bromide was added dropwise to the above solution at -40°C, and reacted at room temperature for 15 h. After filtration, the solid was sucked dry, and the filtrate was concentrated to 5 mL to obtain colorless crystals. The mass of solid and crystals was 1.49 g, and the yield was 94%. M.p. 271-273°C. NMR spectrum: 1 HNMR (600MHz, C 6 D. 6 ):δ6.99-6.91(m,6H,Ar-H),4.88(s,1H,CH),3.12(s,4H,OCH 2 CH 3 ),2.65(s,6H,CH 3 ),2.08(s,6H,CH 3 ),1.55(s,6H,NCCH 3 ),0.48(s,6H,OCH 2 CH 3 ) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ):δ168.87 (NCCH 3 ), 147.75, 131.57, 129.56, 124.76 (Ar-C), 95.31 (=CH), 65.96 (OCH 2 CH 3 ),23.52(OCH 2 CH 3 ), 21.09 (NCCH 3 ), 18.89, 13.15 (CH 3 ) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com