Method for differentiating human multipotential stem cells into hematopoietic stem cells and culture additive
A technology for hematopoietic stem cells and additives, which is applied in cell culture active agents, artificially induced pluripotent cells, biochemical equipment and methods, etc. stability issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1. Differentiation of human pluripotent stem cells to hematopoietic stem cells
[0130] Two types of human pluripotent stem cells were used in this example, namely H1 cells and CD34-iPSC cells.
[0131] 1. Inoculate human pluripotent stem cells in a 6-well plate (2.5×10 per well 5 cells) were cultured in TeSR-E8 medium at 37°C until the cell confluency was 70%-80%.
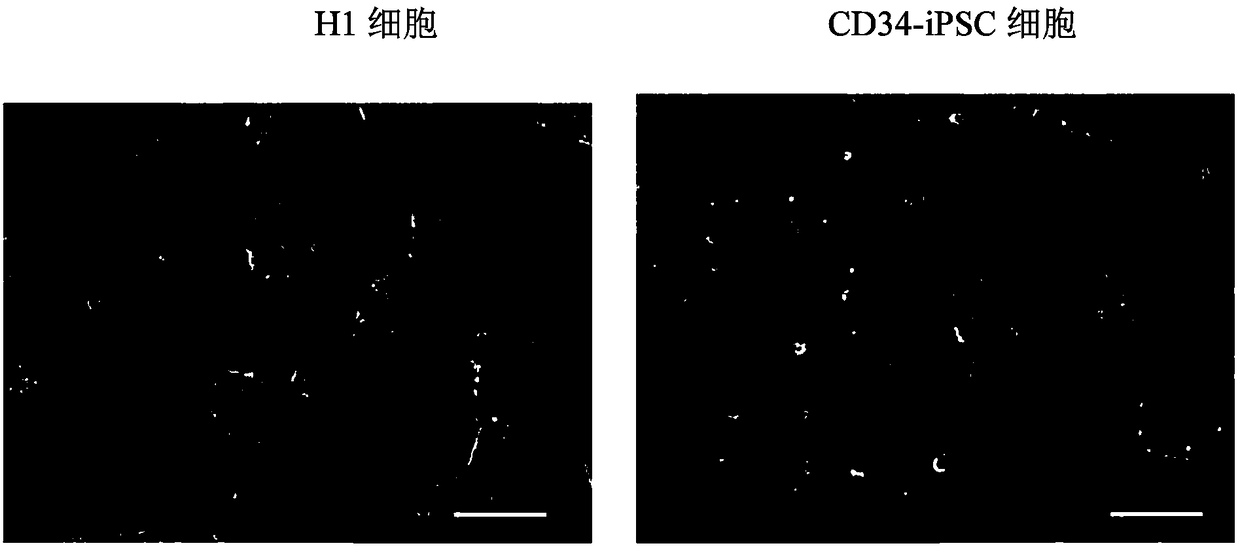
[0132] 2. After completing step 1, take the six-well plate, suck off the culture supernatant, and add PBS buffer preheated to 37°C to wash twice. At this point, the morphology of H1 cells and CD34-iPSC cells is shown in figure 1 (Scale bar 100 μm).
[0133] 3. After completing step 2, take the six-well plate, add 1ml of cell digestion solution Accutase to each well, let it stand at 37°C for 3-5min, then add an appropriate amount of RPMI 1640 basal culture medium to stop the digestion, and collect the cells by centrifugation.
[0134] 4. Inoculate the cells collected in step 3 on a petri dish (th...
Embodiment 2
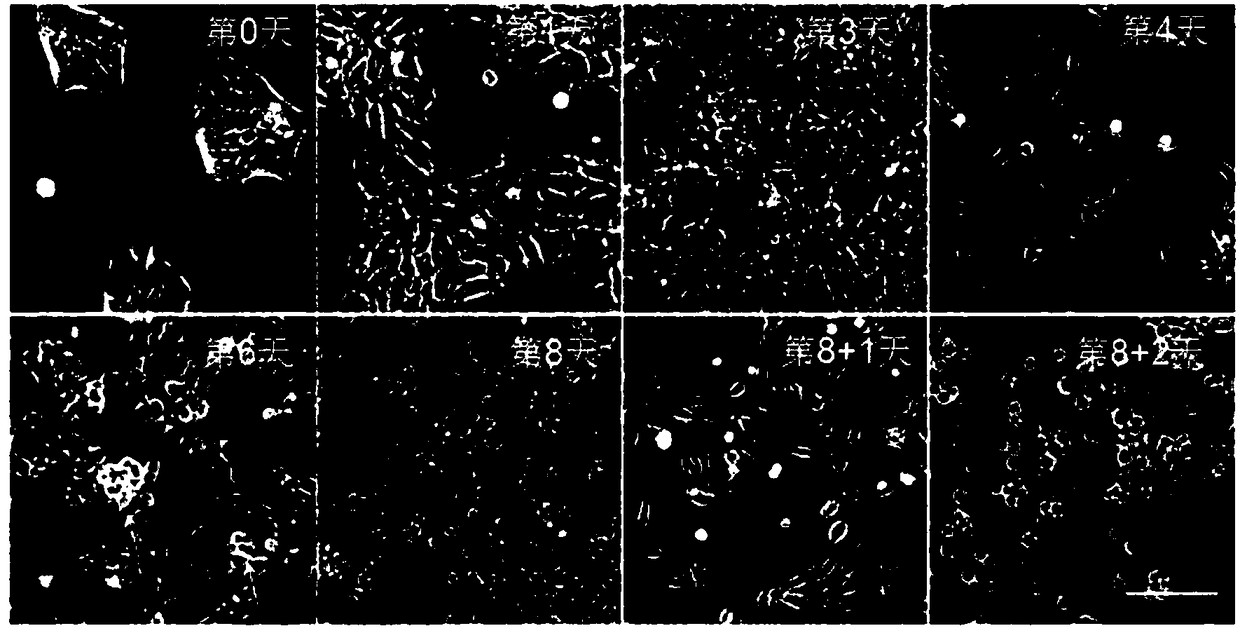
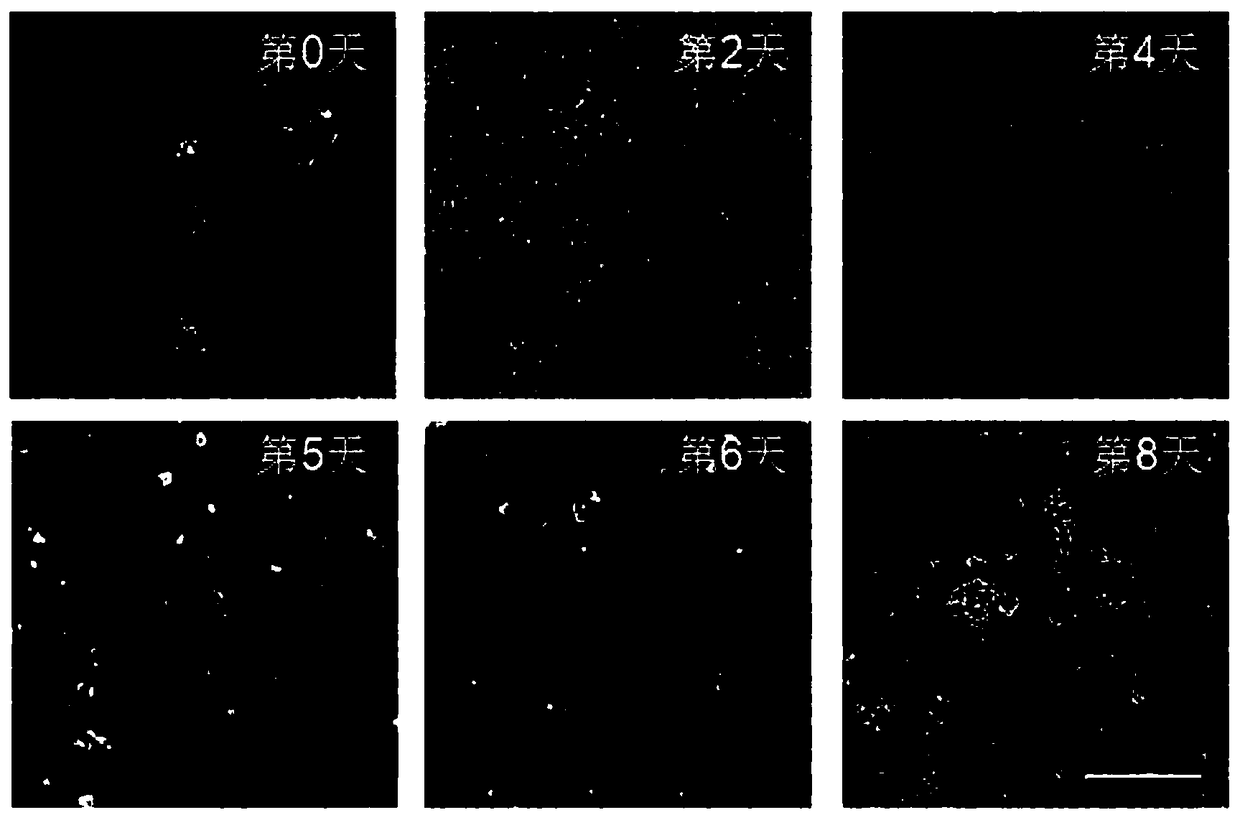
[0143] Example 2, Detection of cells in the process of differentiation of pluripotent stem cells to hematopoietic stem cells
[0144] 1. Detection of cell B
[0145] 1, get the cell B that step 8 obtains in embodiment 1, adopt the PBS damping fluid resuspended cell that contains 5% (v / v) fetal bovine serum to obtain cell suspension (containing 1 * 10 5 cells).
[0146] 2. Add PE-labeled FLK1 antibody to the cell suspension in step 1 (set PE-labeled IgG antibody instead of PE-labeled FLK1 antibody as a negative control), incubate at room temperature in the dark for 20 minutes (during this period, mix well every 5 minutes) once); then washed twice with PBS buffer containing 5% (v / v) fetal bovine serum, and centrifuged to collect the cells.
[0147] 3. After completing step 2, use 500 μL of PBS buffer containing 5% (v / v) fetal bovine serum to resuspend the cells, and use flow cytometry to detect.
[0148] For the results of the detection of mesoderm precursor cells obtained fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com