Tspo fluorescent imaging probe and its synthesis method and application
A probe and fluorescence technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of low expression level and achieve the effect of novel molecular structure and good TSPO affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The synthetic method of TSPO fluorescence imaging probe of the present invention, comprises the following steps:
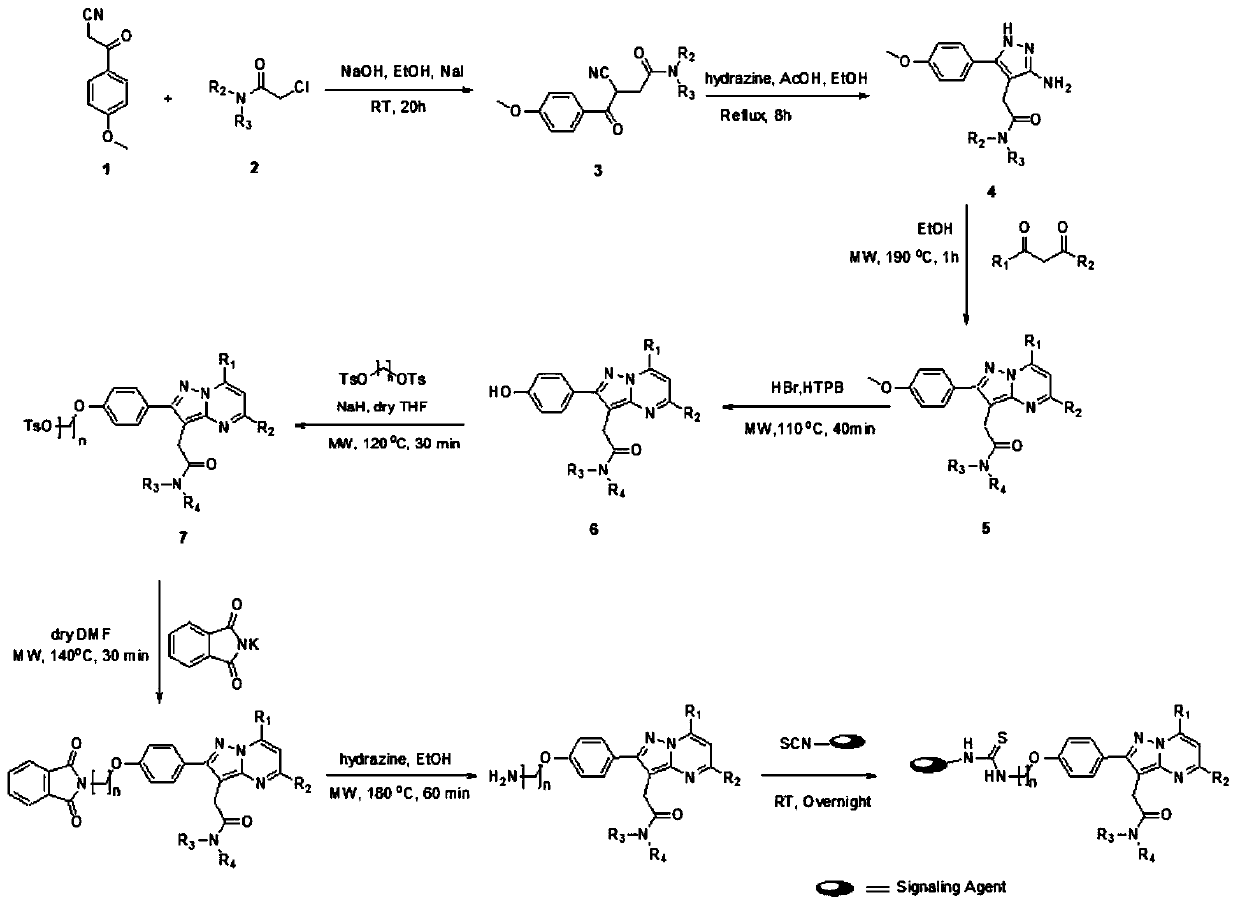
[0031] Using a synthetic method similar to that reported in the literature (Tang et al, Tetrahedron Letters 51(35), 4595, Tang et al, Journal of medicinal chemistry 56(8), 3429, Tang et al, Molecular Imaging and Biology (2016)), A variety of compounds 6 were synthesized (such as figure 1 shown). In this reaction process, the microwave synthesis method (MAOS) is mainly used, which can greatly improve the reaction speed and yield. Using the synthesized compound 6, a long linear alkyl substituent was added to its phenolic hydroxyl end, and compound 7 was synthesized, and a compound 9 with a free amino group was generated through a two-step reaction. The last step of the reaction was to Compound 9 reacted with fluorescent or small dye molecules (with isothiocyanate group) to generate new fluorescent probes TDW-F-1, TDW-F-2, TDW-F-3.
[0032] Different compou...
Embodiment 1
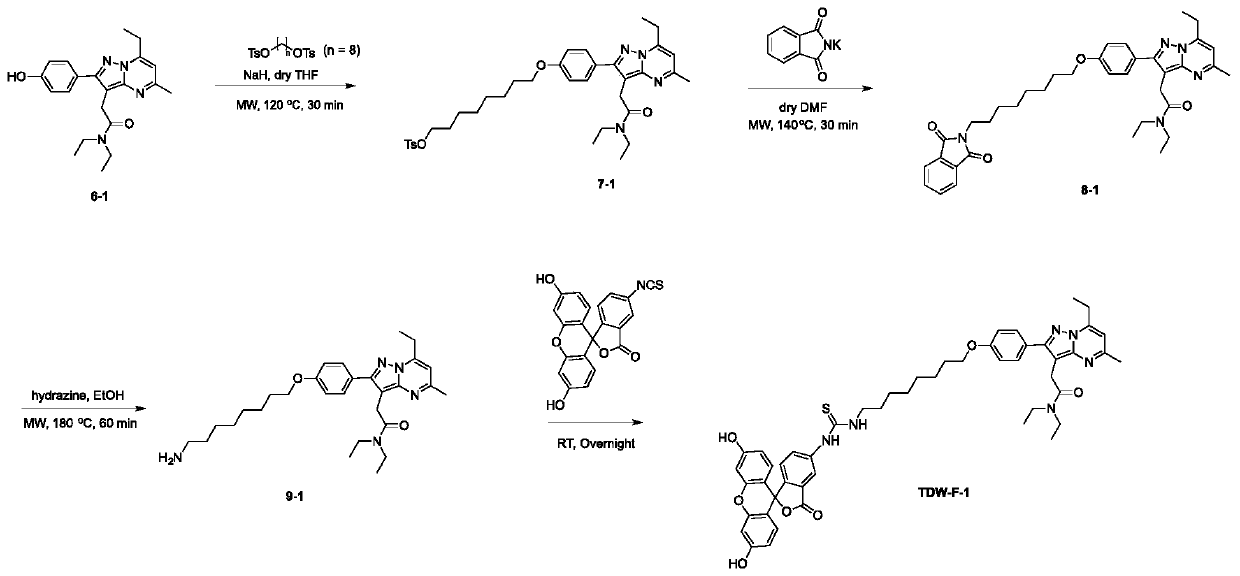
[0034] The synthetic method of embodiment 1, TDW-F-1 (as figure 2 ):
[0035] (1) Synthesis of compound 7-1
[0036]6-1 (40mg, 0.109mmol) was dissolved in 5mL of anhydrous tetrahydrofuran, then NaH (7.9mg, 0.327mmol) was added under ice-bath conditions, after 30 minutes of reaction, octane-1,8-diyl bis( 4-methylbenzenesulfonate) (148mg, 0.327mmol), and then the reaction system was placed in a microwave reaction system and heated at 120°C for 30min. The progress of the reaction was detected by mass spectrometry. When the reaction was finished, it was neutralized with 0.5M HCl (50 mL), and extracted with dichloromethane (50 mL*3). Then it was separated by column chromatography (separation condition: DCM / MeOH=95 / 5 (V / V)), and finally compound 7-1 was obtained (yellow solid, 50 mg, 70% yield).
[0037] (2) Synthesis of compound 9-1
[0038] Compound 7-1 (50mg, 0.076mmol) and potassium 1,3-dioxoisoindolin-2-ide (28mg, 0.15mmol) were added to 3mL DMF, and after they were dissol...
Embodiment 2
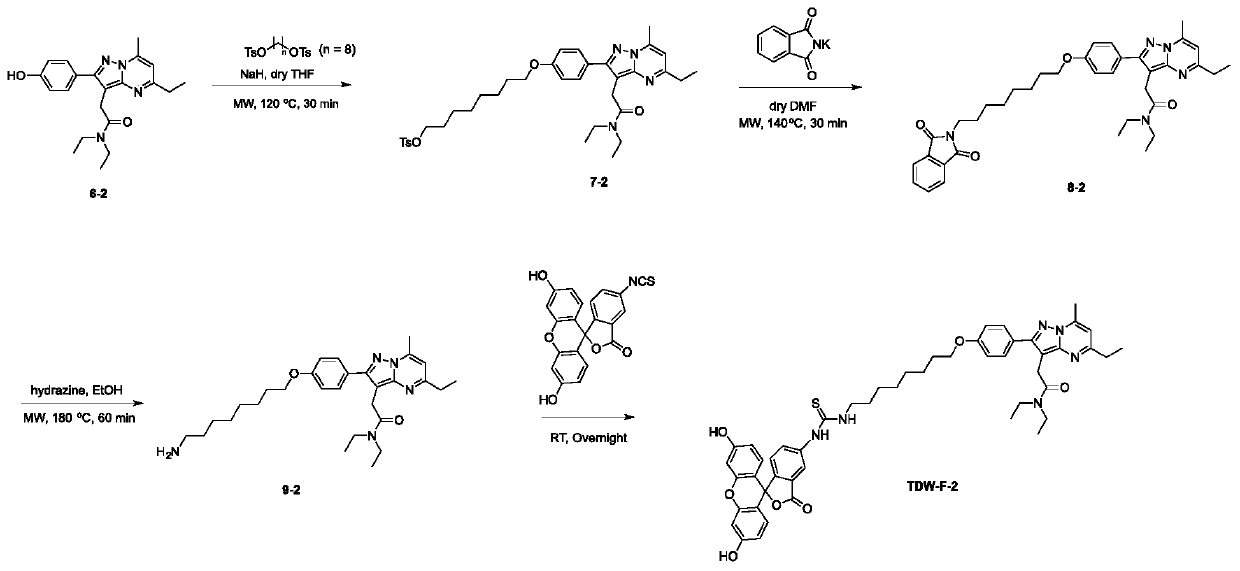
[0041] The synthetic method of embodiment 2 TDW-F-2 (as image 3 ):
[0042] (1) Synthesis of compound 7-2
[0043] 6-2 (40mg, 0.109mmol) was dissolved in 5mL of anhydrous tetrahydrofuran, then NaH (7.9mg, 0.327mmol) was added under ice-bath conditions, after 30 minutes of reaction, octane-1,8-diyl bis( 4-methylbenzenesulfonate) (148mg, 0.327mmol), and then the reaction system was placed in a microwave reaction system and heated at 120°C for 30min. The progress of the reaction was detected by mass spectrometry. When the reaction was finished, it was neutralized with 0.5M HCl (50 mL), and extracted with dichloromethane (50 mL*3). Then it was separated by column chromatography (separation condition: DCM / MeOH=95 / 5 (V / V)), and finally compound 7-1 was obtained (yellow solid, 52 mg, 74% yield).
[0044] (2) Synthesis of compound 9-2
[0045] Compound 7-2 (50mg, 0.076mmol) and potassium 1,3-dioxoisoindolin-2-ide (28mg, 0.15mmol) were added to 3mL DMF, and after they were dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com