A kind of polypeptide and its application
A polynucleotide and amino acid technology, applied in the field of polypeptides and their applications, can solve the problems of limited, unclear, and increased p53
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
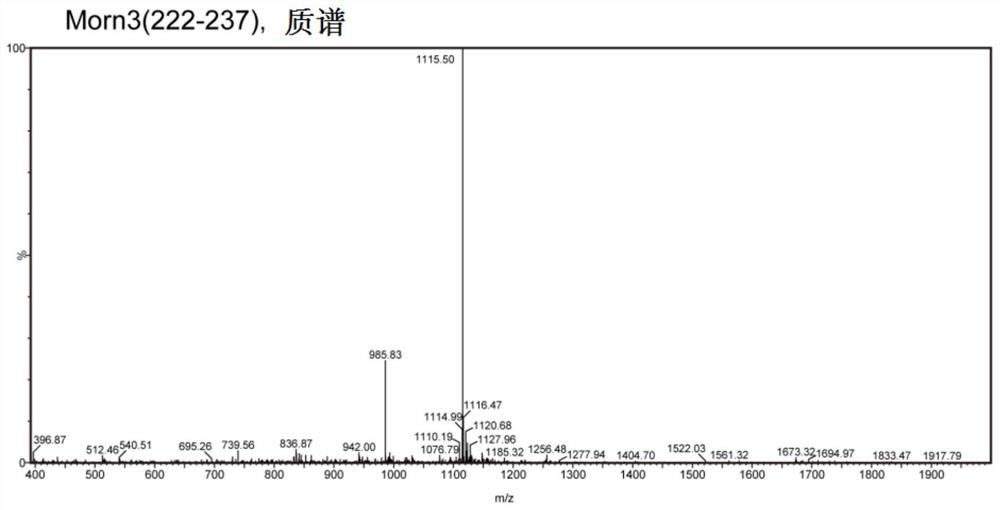
[0034] The basic procedure for the preparation of the polypeptide involved in the present invention is as follows: first, an amino acid protected by an Fmoc group is connected to an insoluble solid-phase carrier Wang resin, and then the protecting group of the amino group is removed, and the first amino acid is connected to the solid phase carrier Wang resin. Secondly, the carboxyl group of the second amino acid whose amino group is blocked is activated by a condensing agent, and the second amino acid whose carboxyl group is activated reacts with the amino group of the first amino acid that has been connected to the solid phase carrier to form a peptide bond, thereby A dipeptide with a protecting group is formed on the solid support. Repeat the above peptide bond formation reaction to make the peptide chain grow from the C-terminus to the N-terminus until the desired length of the peptide chain is reached. Finally, the ester bond between the peptide chain and the solid phase c...
Embodiment 2
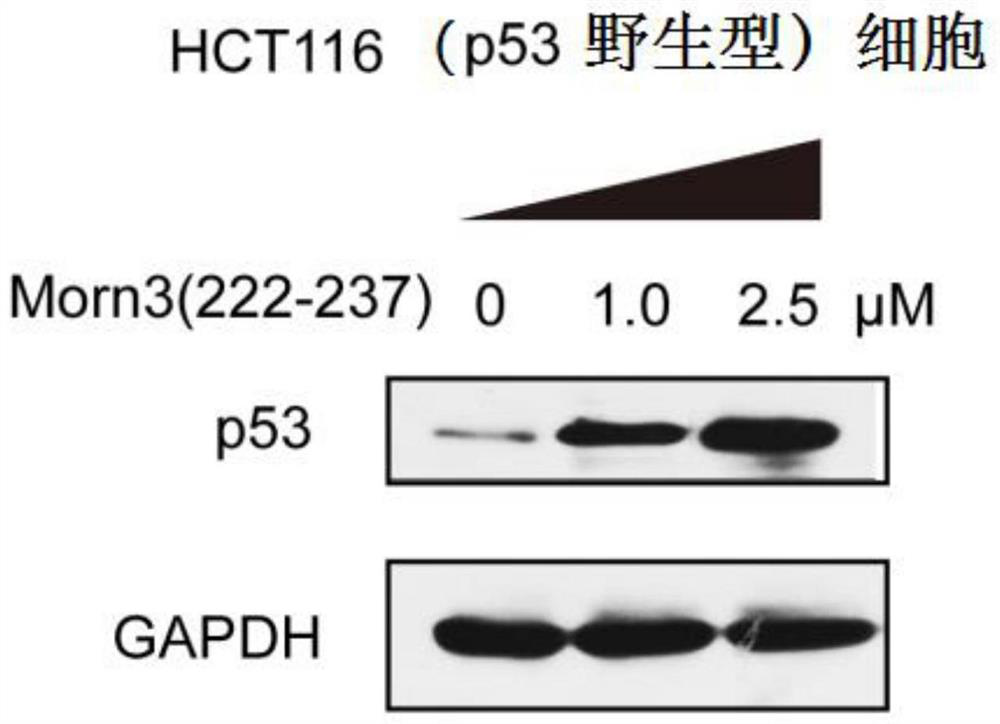
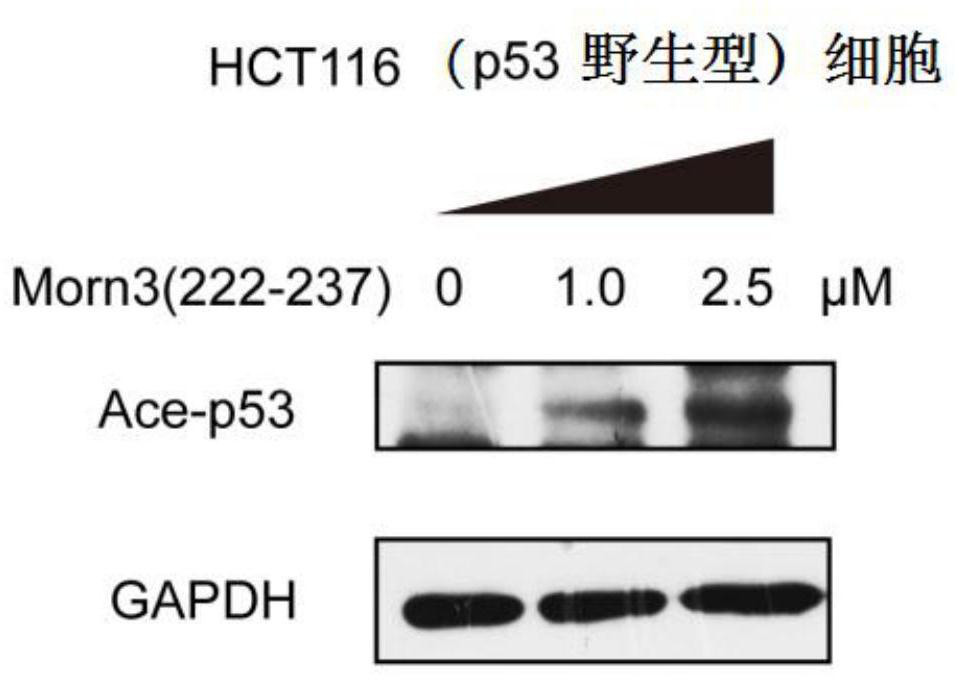
[0039] In vitro culture of tumor cells: Human colorectal cancer tumor cells HCT116 (expressing wild-type p53, purchased from ATCC) or other cells were cultured in vitro according to conventional methods, using DMEM or RPMI1640 medium containing 10-20% calf serum, Cultivate in an incubator containing 5% carbon dioxide at 37 degrees Celsius, and subculture when the cell density reaches 80%-90%.
[0040] The administration method of Morn3(222-237) polypeptide: (1) Culture the above-mentioned cells with 6-well plates, when the cell density reaches 50-60%, add Morn3(222-237) polypeptide to the culture medium, until the final The concentration is 1 μM to 2.5 μM or other concentrations, and continue to incubate in the incubator for more than 4 hours.
[0041] Western Blot experiment was used to detect the effect of Morn3 (222-237) polypeptide on p53 protein expression and acetylation level: add 0.1 ml of RIPA lysate (containing protease inhibitors) to each well of the 6-well plate co...
Embodiment 3
[0043] RT-qPCR experiment to detect the effect of Morn3(222-237) polypeptide on the transcriptional activation of p53 downstream genes: Morn3(222-237) polypeptide (or PBS, control polypeptide) was added to HCT116 cells to a final concentration of 2.5 μM, and a total of After culturing for 48 hours, total RNA was extracted, and cDNA was obtained by reverse transcription with random primers. Use p21, BAX, PUMA and other p53 downstream gene-specific primers for qPCR amplification to detect changes in the transcriptional activation ability of p53 (see: Xu J, et al., CellRep.2013; 3(5):1526-38). After Morn3(222-237) polypeptide was co-cultured with cells, the transcriptional activation of p21, BAX, and PUMA was significantly increased, as shown in Figure 4 shown. Figure 4 The vertical axis in is the ratio of other treatment groups with PBS as 1.0. Figure 4-6 The asterisks in * represent statistical test P<0.01 (two-sided T test), and there are significant differences between t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com