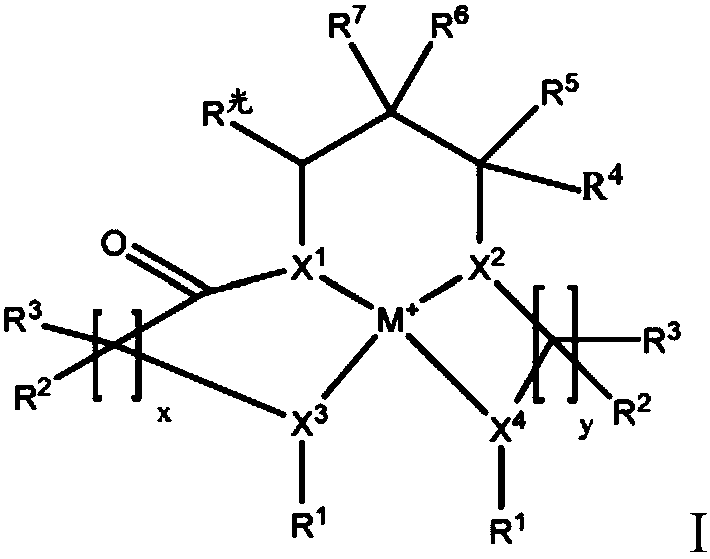
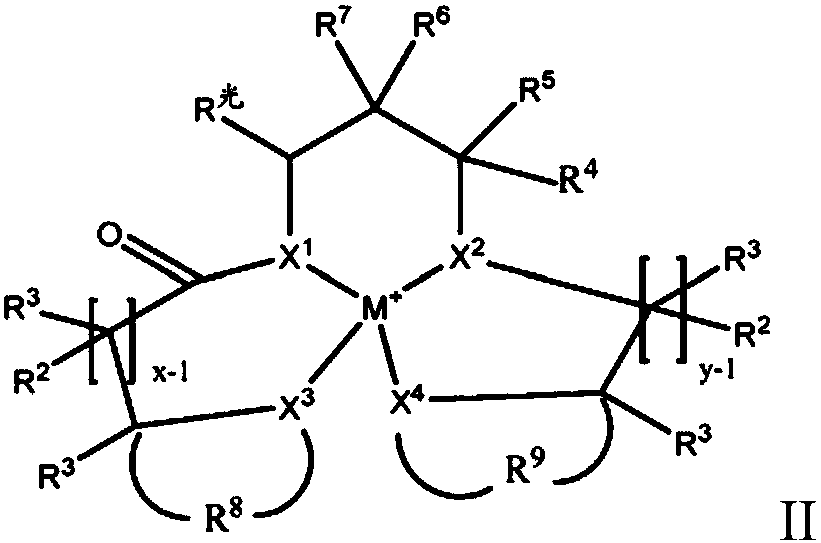
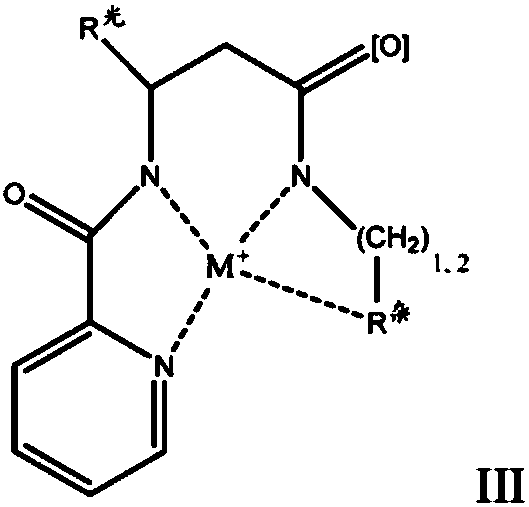
Redox polymerizable dental composition with photolabile transition metal complexes
A transition metal, photo-instability technology, applied in dentistry, dental prosthesis, dental preparations, etc., can solve the problems of poor storage stability and premature curing of stress accumulation preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0265] Amounts of materials are expressed by weight or weight percent ("wt %) unless otherwise indicated.
[0266] materials used :
[0267] Acetone (EMD Millipore Corporation, Billerica, MA)
[0268] Alumina, powder, < / = 10 μm average particle size (Sigma-Aldrich, St. Louis, MO)
[0269] 3-Amino-3-(2-nitrophenyl)propanoic acid, 98% (Alfa Aesar, Ward Hill, MA)
[0270] Ammonium chloride (EMD Chemicals, Inc. Gibbstown, NJ)
[0271] BENZOFLEX 9-88 plasticizer, Eastman Chemical Co., Kingsport, TN (Eastman Chemical Co., Kingsport, TN)
[0272] Benzyltributylammonium chloride (Sigma-Aldrich, St. Louis, MO)
[0273] BisGMA: 2,2-bis[4-hydroxy-3-methacryloyloxy)propoxyphenyl]propane (Sigma Aldrich, St.Louis, MO) )
[0274] CAB-O-SIL TS720 (Cabot Corporation, Billerica, MA)
[0275] CDCl 3 : Deuterochloroform (Cambridge Isotope Laboratories, Andover, MA)
[0276] CH 2 Cl 2 : Dichloromethane (EMD Millipore Corporation, Billerica, MA)
[0277] CHP: cumene hydroperoxide, 80%...
preparation example 1
[0314] Preparation Example 1 (PE-1): Copper Complex
[0315] The assigned copper metal complexes are according to Ciesienski, K.L; Haas, K.L.; Dickens, M.G.; Tesema, Y.T.; Franz, K.J. "A Photolabile Ligand for Light-Activated Release of Caged Copper" J.Am.Chem.Soc.2008 , vol.130, pages 12246-12247 (“Photolabile Ligands for Photoactivated Release of Copper Encapsulation”, Journal of Chemical Science Abstracts, 2008, Vol. 130, Pages 12246-12247) prepared.
[0316]
preparation example 2
[0317] Preparation Example 2 (PE-2): Copper Complex
[0318] The copper metal complexes were developed according to Ciesienski, K.L; Haas, K.L.; Franz, K.J. "Development of Next-generation Photolabile Cages with Improved Copper Binding Properties" Dalton Trans. 2010, vol.39, pages 9538-9546 ("with improved Development of next-generation photolabile cages with copper-binding properties", Acta Dalton, 2010, Vol. 39, pp. 9538-9546) preparation.
[0319]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com