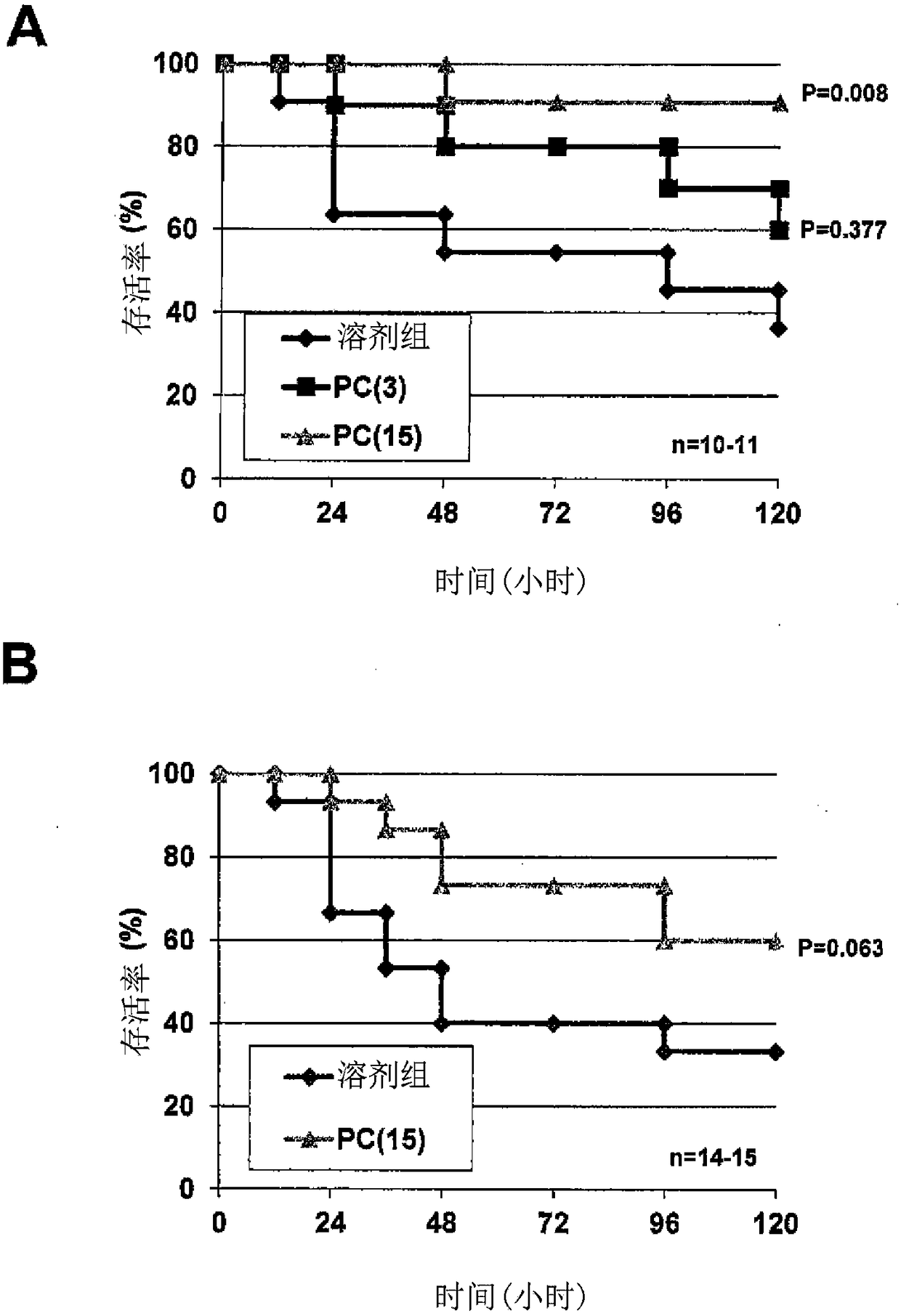
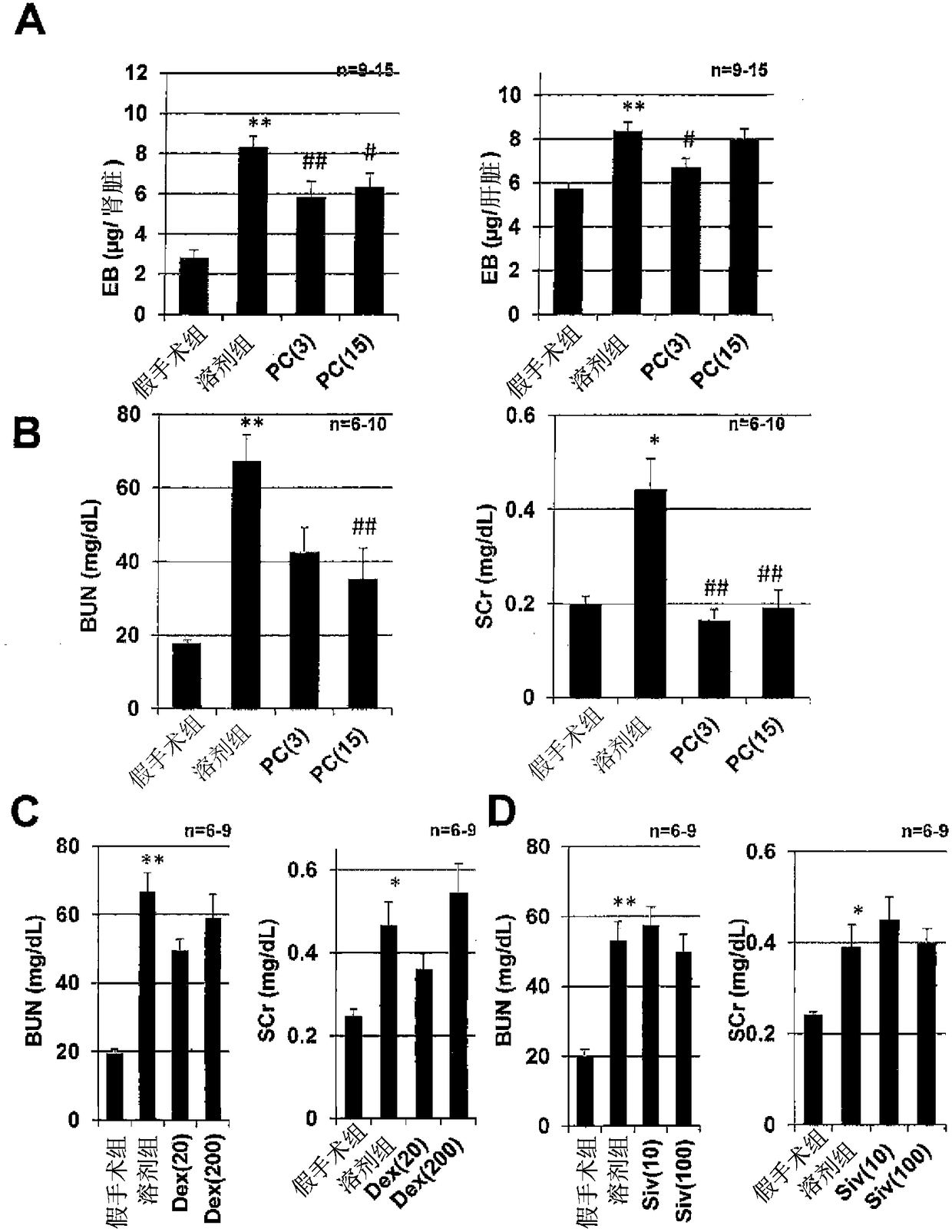
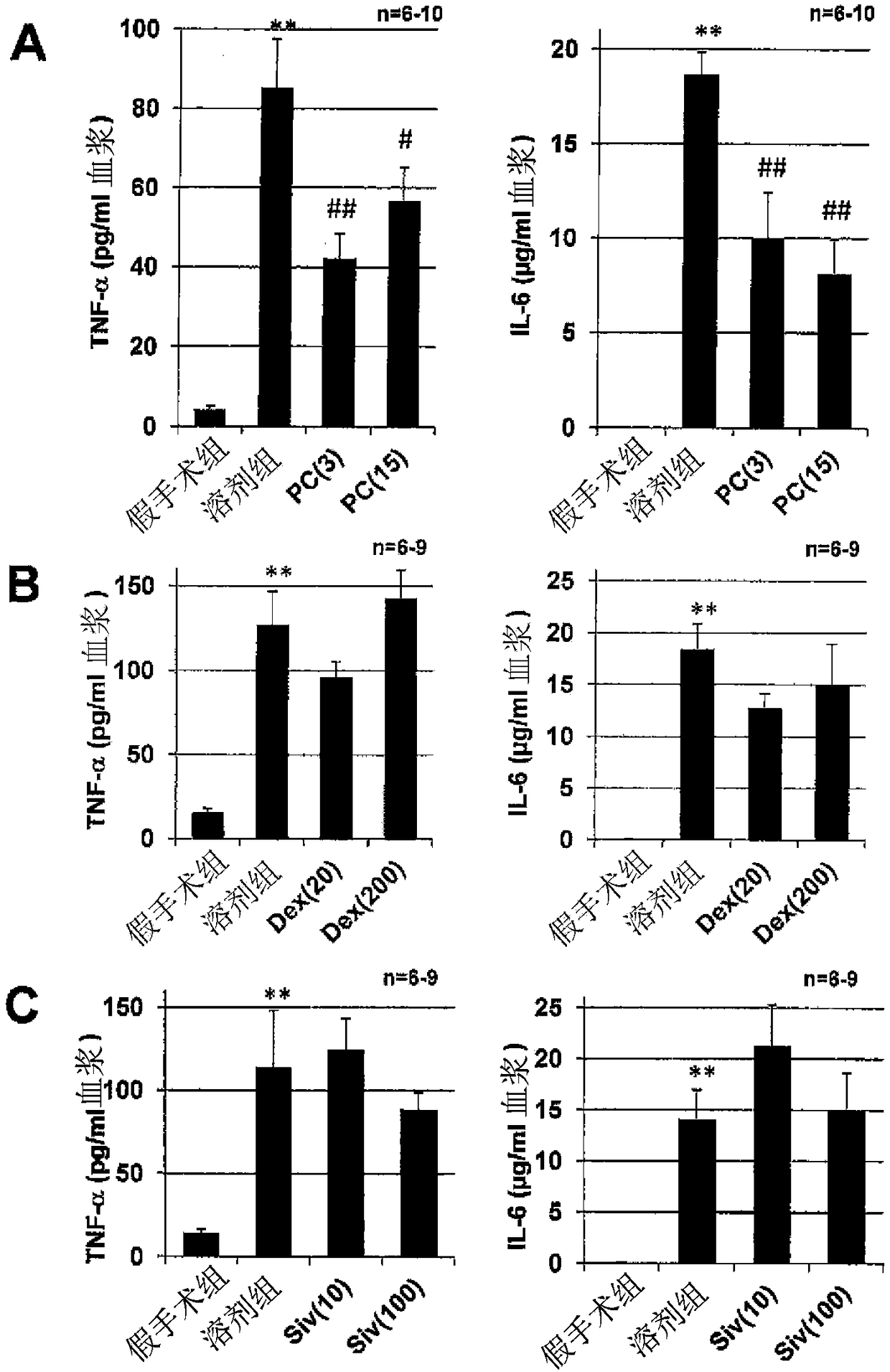
Acute respiratory distress syndrome therapeutic agent
A technology for acute respiratory distress syndrome, applied in the field of acute respiratory distress syndrome therapeutic agents, which can solve the problems of low stability and low tissue affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0105] Hereinafter, the present invention will be described in more detail by referring to test examples specifically studied by the present inventors by substituting Examples, but the present invention is not limited to these descriptions.
[0106]
[0107] LPS (lipopolysaccharide: lipopolysaccharide) was obtained from Escherichia coli and purchased from Sigma (St Louis, MO).
[0108] Diff-Quik was purchased by Sysmex Corporation (Kobe).
[0109] DRI-CHEM test pieces (for BUN identification) were purchased from Fujifilm Corporation.
[0110] L-012 (luminescent probe), LabAssay Creatinine Quantitative Kit, and Evans Blue were purchased from Wako Pure Chemical Industries.
[0111] Novo-Heparin (5000 units) was purchased by Mochida Pharmaceutical Company.
[0112] Pentobarbital was purchased from Tokyo Chemical Company.
[0113] ICR mice (6-7 weeks old) were purchased from Charles River Company.
[0114]
[0115] The mice were anesthetized with pentobarbital (10 mg / kg, i...
preparation example 1
[0227] Preparation Example 1: Intravenous Injection
[0228] 1% (w / w) PC-SOD, 10% (w / w) sucrose, and 0.05% (w / w) benzalkonium chloride were dissolved in 5% xylitol aqueous solution, and freeze-dried. To the obtained freeze-dried preparation, 0.5% carboxymethylcellulose or water for injection, which was separately filled in a vial, was added, whereby an intravenous injection preparation was obtained.
Embodiment 2
[0229] Example 2: Inhalation
[0230] Liquids for Inhalation (1)
[0231] A solution for inhalation was prepared by dissolving 1% (w / w) PC-SOD, 10% (w / w) sucrose, and 0.05% (w / w) benzalkonium chloride in 5% xylitol aqueous solution.
[0232] Liquids for Inhalation (2)
[0233] With 1% (w / w) PC-SOD, 10% (w / w) sucrose, 0.05% (w / w) benzalkonium chloride, 10% (w / w) polyethylene glycol, 20% (w / w) w) Propylene glycol and the remaining amount of pure water are used to prepare a liquid for inhalation.
[0234] powder for inhalation
[0235] A powder for inhalation was prepared with 5% (w / w) PC-SOD and the balance sucrose (fine powder).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com