Application of dihydrotanshinone I in preparation of medicines for treating multi-drug resistant tumors, and preparation method of dihydrotanshinone I
A multi-drug resistance, dihydrotanshinone technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems that have not yet been found, achieve good clinical application prospects, inhibit the growth of multi-drug resistance tumors, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation process of dihydrotanshinone I
[0054] (1) After the salvia miltiorrhiza crude drug is pulverized, get 20 kilograms of pulverized medicinal materials and add 10 times the amount of sherwood oil to soak and extract, collect the filtrate after filtration and recycle, and reclaim the solvent to obtain 200 g of crude extract I;
[0055] (2) After mixing the crude extract I with the C18 reverse phase chromatography filler, the mobile phase was eluted with a 20%-60% acetonitrile aqueous solution with a gradient of 10 column volumes, and the target site was collected to obtain 26 g of the crude extract II;
[0056] (3) Utilize the normal phase chromatography silica gel column of the crude extract II, and use petroleum ether as the mobile phase: ethyl acetate=(1:9)-(3:2) to elute with a 10-fold column volume gradient to obtain dihydrotanshinone I 10g.
Embodiment 2
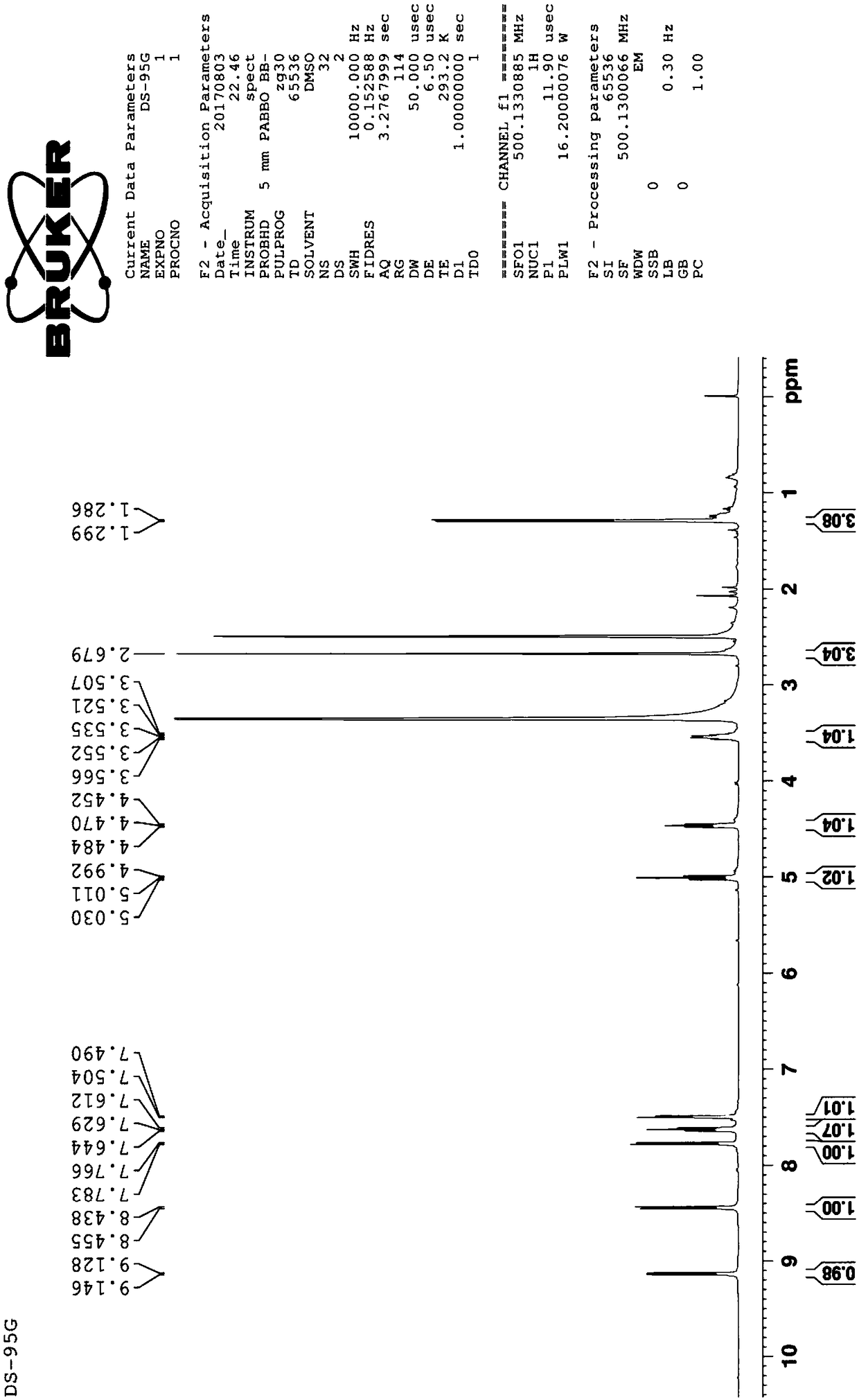
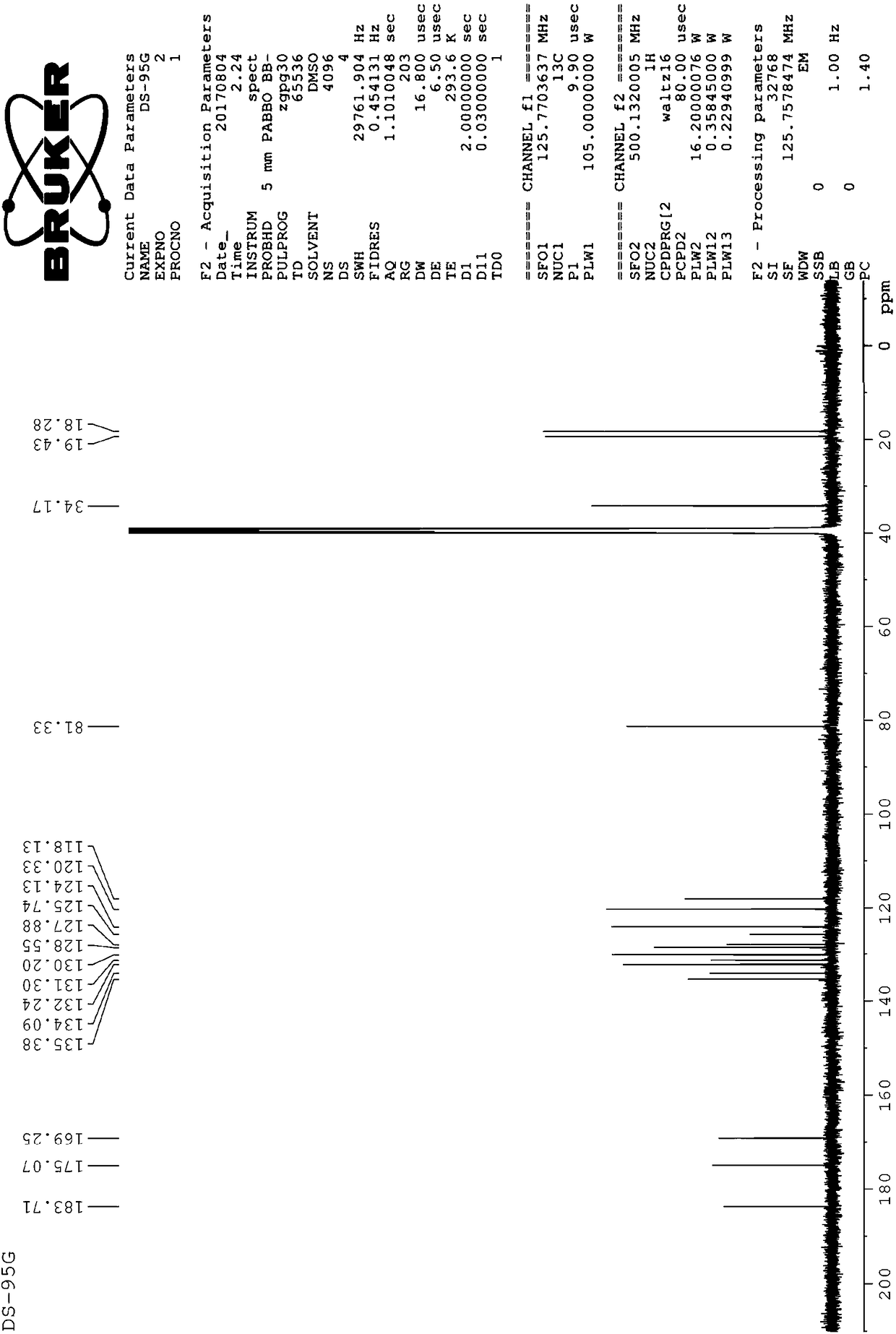
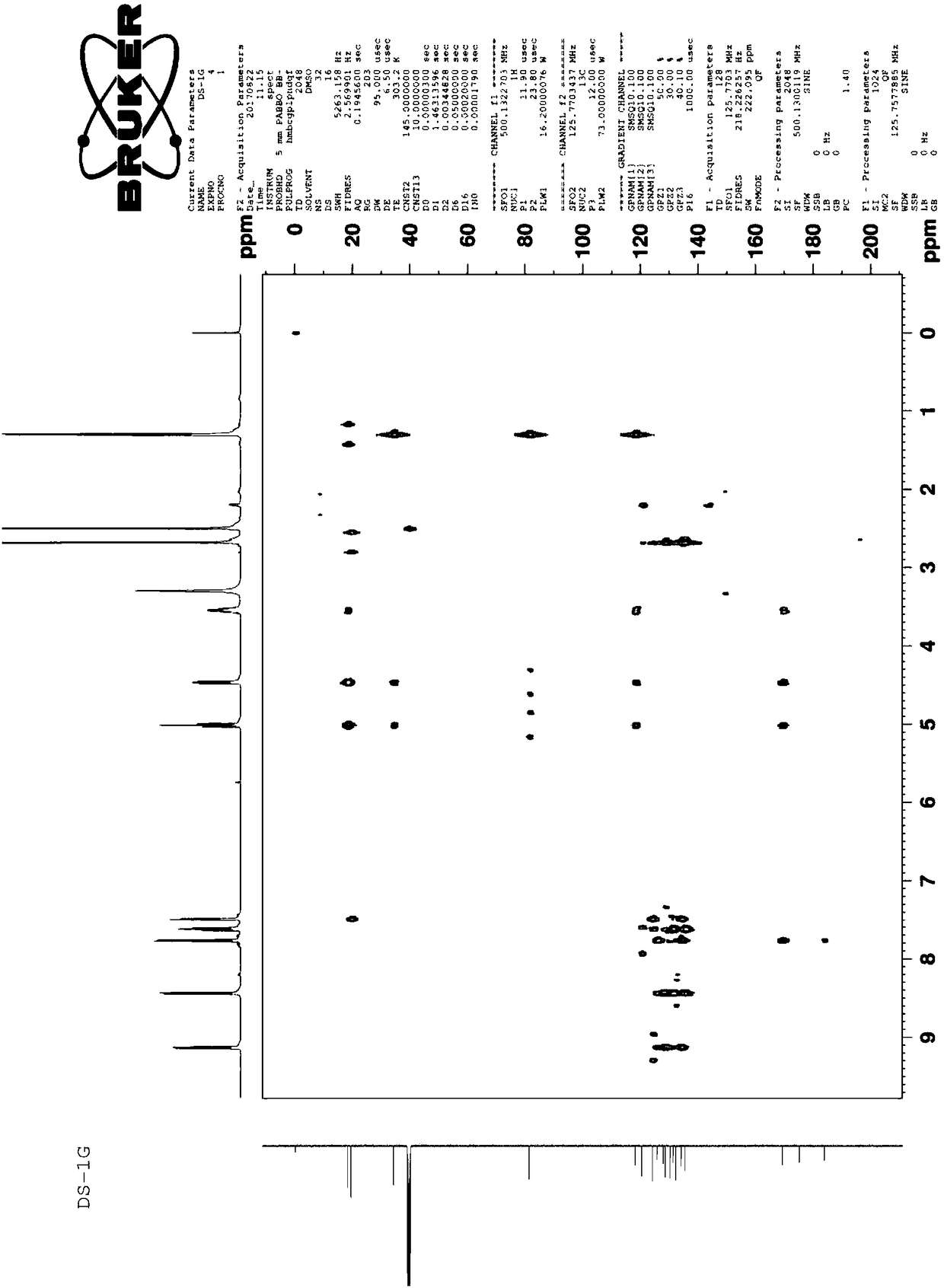
[0057] Example 2: Structural characterization of prepared dihydrotanshinone I
[0058] 2.1 Detection of the melting point of dihydrotanshinone I.
[0059] Take an appropriate amount of the sample obtained in Example 1, place it in a capillary tube for melting point determination, lightly tap the tube wall, place it vertically on a watch glass, put the capillary tube from the upper mouth and let it fall freely, repeat several times, so that the powder is tightly assembled in the capillary tube. Melt capped. The height of the loaded sample is 3 mm.
[0060] In addition, put the thermometer into the container containing the temperature transfer liquid, so that the distance between the bottom of the mercury bulb of the thermometer and the bottom of the container is more than 2.5cm; place. Heat the temperature-transfer fluid, and when the temperature rises to about 10°C lower than the specified lower melting point, immerse the capillary with the sample in the temperature-transfe...
Embodiment 3
[0068] Example 3: Comparison of anti-multidrug-resistant cell proliferation inhibition effects of dihydrotanshinone I and paclitaxel and other drugs
[0069] Experimental method: The multidrug-resistant cell MCF-7 / MDR and its parental cell MCF-7 were used as objects to investigate the effects of drugs such as dihydrotanshinone I and paclitaxel on the inhibition of cell proliferation.
[0070] Two kinds of cells in the growth phase with good growth and shape were taken, and 10 5 The number of cells per well was placed in a 96-well plate, 100 μL per well. After being incubated in an incubator for 18-24 hours to adhere to the wall, the MCF-7 cell group was added with each liquid sample, and the final concentrations were 4.5, 2.25, 1.13, 0.56, and 0.28 μmol / L; the MCF-7 / MDR group was added with The final concentration of the drug solution sample is shown in Table 2. For each concentration, 3 parallel wells were paralleled. After incubation in the incubator for 48 hours, the mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com