Preparation method for single-phase crosslinked sodium hyaluronate gel
A technology of cross-linking hyaluronic acid and sodium hyaluronate, which is applied in the direction of medical preparations and pharmaceutical formulas of non-active ingredients, and can solve the problem of uneven mixing of biphasic HA, differences in the pushing force of preparations, and unstable quality control and other issues to achieve the effect of reducing the risk of cross-contamination, reducing manual operations, and reducing the risk of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
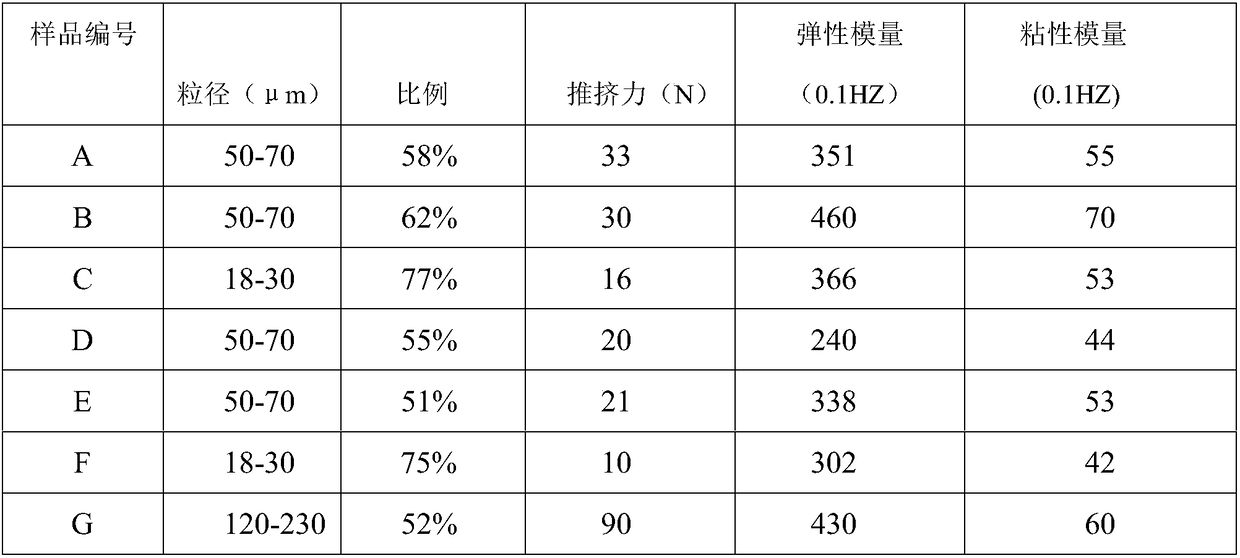
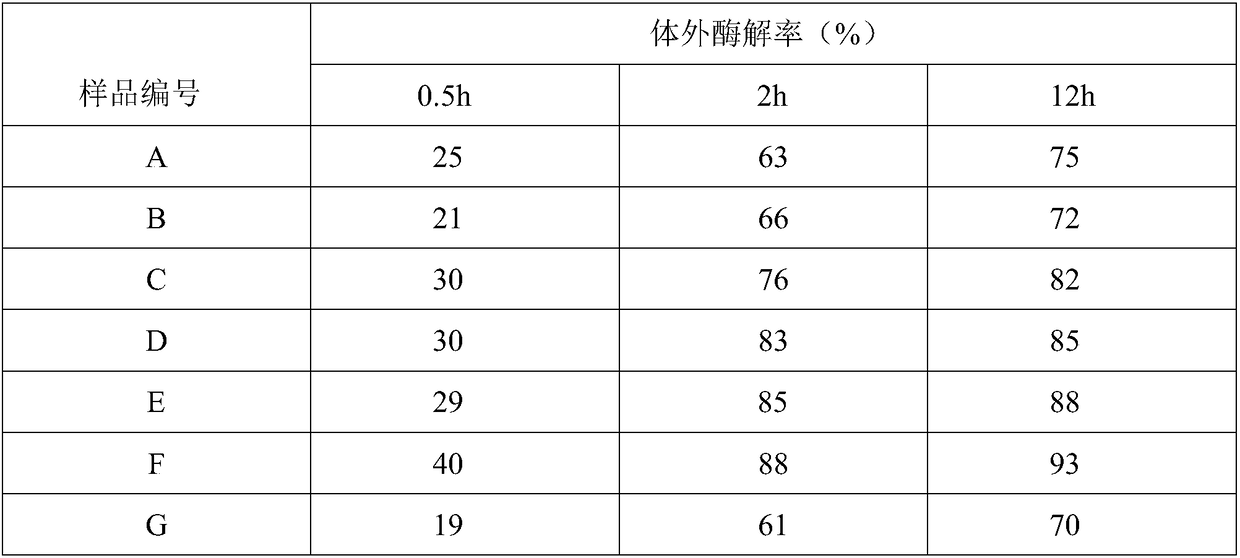
[0041] Prepare 100ml of 0.32mol / L sodium hydroxide solution, add 750μL of BDDE, mix well for later use, take 8.0g of sodium hyaluronate raw material at room temperature, mix with the above solution, stir and dissolve until there is no visible white undissolved to the naked eye HA so far. Adjust the temperature of the water bath to 40°C, keep the dissolved HA in the water bath for 3 hours, then take it out, divide the cross-linked HA gel into square blocks with a size of 1-2 cm, and set aside. Prepare a physiological balance solution (containing sodium chloride, sodium dihydrogen phosphate, disodium hydrogen phosphate, water for injection), add the block gel, stir at low speed to swell, replace the buffer at intervals of 1h, 6h and 16h, 10L each time, Stop dialysis until the pH of the buffer reaches neutral and the weight of the gel is about 400 g, reaching the end point of dialysis. The dialyzed gel was taken out, sieved twice with a sieve and preliminarily crushed to obtain ...
Embodiment 2
[0043] Prepare 100ml of 0.32mol / L sodium hydroxide solution, add 750μL of BDDE, mix well for later use, take 8.0g of sodium hyaluronate raw material at room temperature, mix with the above solution, stir and dissolve until there is no visible white undissolved to the naked eye HA so far. Adjust the temperature of the water bath to 60°C, keep the dissolved HA in the water bath for 1 hour, then take it out, divide the cross-linked HA gel into square blocks with a size of 1-2 cm, and set aside. Prepare a physiological balance solution (containing sodium chloride, sodium dihydrogen phosphate, disodium hydrogen phosphate, water for injection), add the block gel, stir at low speed to swell, replace the buffer at intervals of 1h, 6h and 24h, 10L each time, Stop dialysis until the pH of the buffer reaches neutral and the weight of the gel is about 400 g, reaching the end point of dialysis. The dialyzed gel was taken out, sieved twice with a sieve and preliminarily crushed to obtain a...
Embodiment 3
[0045] Prepare 100ml of 0.32mol / L sodium hydroxide solution, add 750μL of BDDE, mix well for later use, take 8.0g of sodium hyaluronate raw material at room temperature, mix with the above solution, stir and dissolve until there is no visible white undissolved to the naked eye HA so far. Adjust the temperature of the water bath to 60°C, keep the dissolved HA in the water bath for 0.5h, then take it out, divide the cross-linked HA gel into square blocks with a size of 1-2 cm, and set aside. Prepare a physiological balance solution (containing sodium chloride, sodium dihydrogen phosphate, disodium hydrogen phosphate, water for injection), add the block gel, stir at low speed to swell, replace the buffer at intervals of 1h, 6h and 24h, 10L each time, Stop dialysis until the pH of the buffer reaches neutral and the weight of the gel is about 400 g, reaching the end point of dialysis. The dialyzed gel was taken out, sieved twice with a sieve and preliminarily crushed to obtain a g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com