Preparation method for 2,4-dichlorophenoxyacetate missible oil
A technology of dichlorophenoxyacetate and haloacetate, applied in the field of preparation of 2,4-dichlorophenoxyacetate emulsifiable concentrate, which can solve uneven spraying, complicated operation, low product yield, etc. problems, to achieve effective control of exhaust gas and dust, increase reaction capacity, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
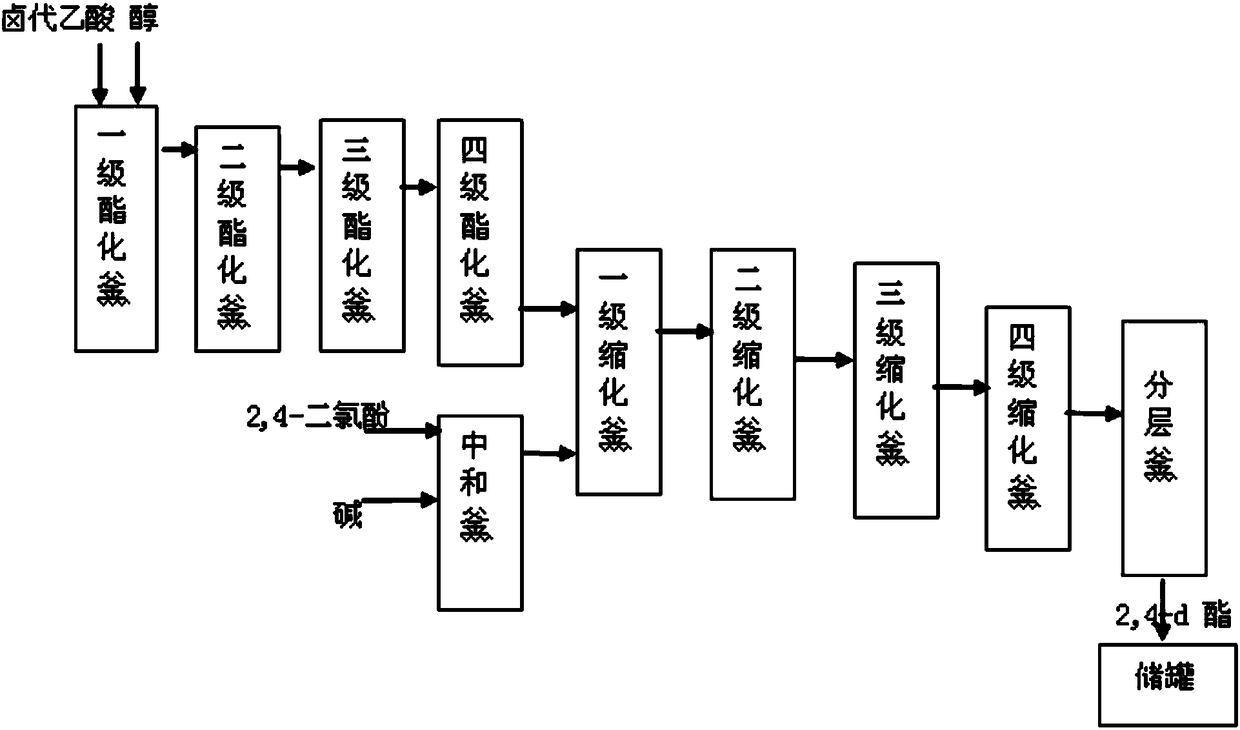
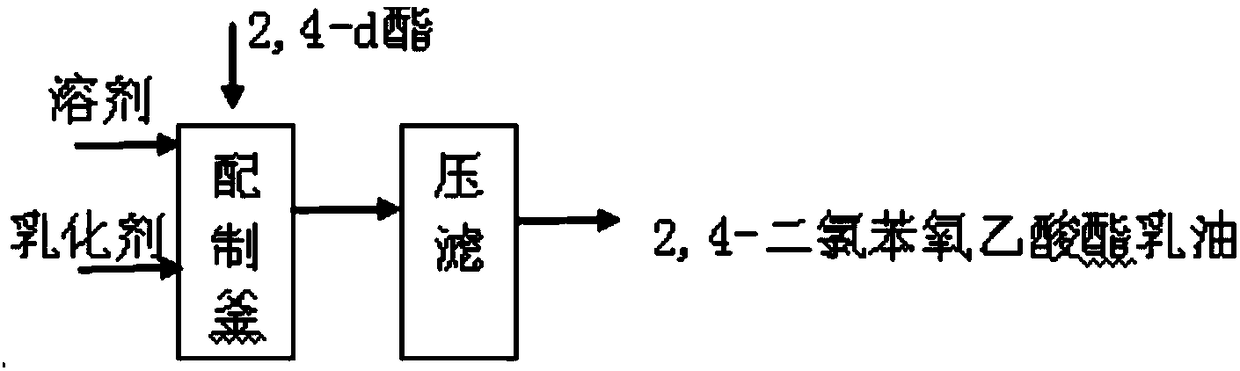
[0020] The invention provides a kind of preparation method of 2,4-dichlorophenoxyacetate emulsifiable concentrate, comprises the steps:
[0021] a) After the haloacetate is mixed with 2,4-dichlorophenoxylate, condensation reaction is carried out, and 2,4-dichlorophenoxyacetate is obtained through layering;
[0022] b) mixing the 2,4-dichlorophenoxyacetic acid ester with a solvent and an emulsifier to obtain 2,4-dichlorophenoxyacetic acid emulsifiable concentrate.
[0023] The preparation method provided by the invention is directly synthesized to esters and then to the production of emulsifiable concentrate products, which can overcome the low reaction capacity, complicated operation, low product yield, unstable product, difficult control of waste gas and dust, and powder drug efficacy in the prior art. Bad question.
[0024] In the embodiment of the present invention, firstly, a haloacetate is prepared by the following method: an alcohol and a haloacetic acid are subjected t...
Embodiment 1
[0042] Put 2000kg of butanol, 950kg (10kmol) of chloroacetic acid and 1kg of concentrated sulfuric acid into the primary esterification tank, mix and stir, desolventize and dehydrate at 80°C for 1 hour, and then enter the secondary and tertiary esterification tanks through overflow in sequence, and the feeding rate is 3000kg / h, and the residence time of each stage is controlled at 1h to obtain butyl chloroacetate.
[0043] 1640kg (10kmol) of 2,4-dichlorophenol and 1250kg (10kmol) of sodium hydroxide aqueous solution with a mass fraction of 32% were mixed and stirred, and the temperature was raised to 70°C to obtain sodium 2,4-dichlorophenate solution.
[0044] Put the 2,4-dichlorophenate sodium solution into the first-stage condensation kettle and mix it with the butyl chloroacetate obtained by esterification, stir at 80°C for 0.5h, and then enter the second, third, and fourth-stage condensation kettles through overflow , the feed rate is 3000kg / h, and the residence time of e...
Embodiment 2
[0047] Mix and stir 2000kg ethanol, 1400kg (10kmol) bromoacetic acid and 1kg concentrated sulfuric acid, desolventize and dehydrate at 100°C for 0.8h, and then enter the second and third stage esterification kettles through the overflow, the feed rate is 2500kg / h, and each stage stays The time was controlled at 0.8h to obtain ethyl bromoacetate.
[0048] 1640kg (10kmol) of 2,4-dichlorophenol and 1122kg (10kmol) of potassium hydroxide aqueous solution with a mass fraction of 50% were mixed and stirred, and the temperature was raised to 70°C to obtain potassium 2,4-dichlorophenolate solution.
[0049] Put the 2,4-dichlorophenate potassium solution into the ethyl bromoacetate obtained by esterification and mix it into the first-stage condensation kettle, stir at 90°C for 0.5h, and then enter the second, third, and fourth-stage condensation kettles in turn through overflow, The feed rate is 2000kg / h, and the residence time of each stage is controlled at 0.5h. Then the condensation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com