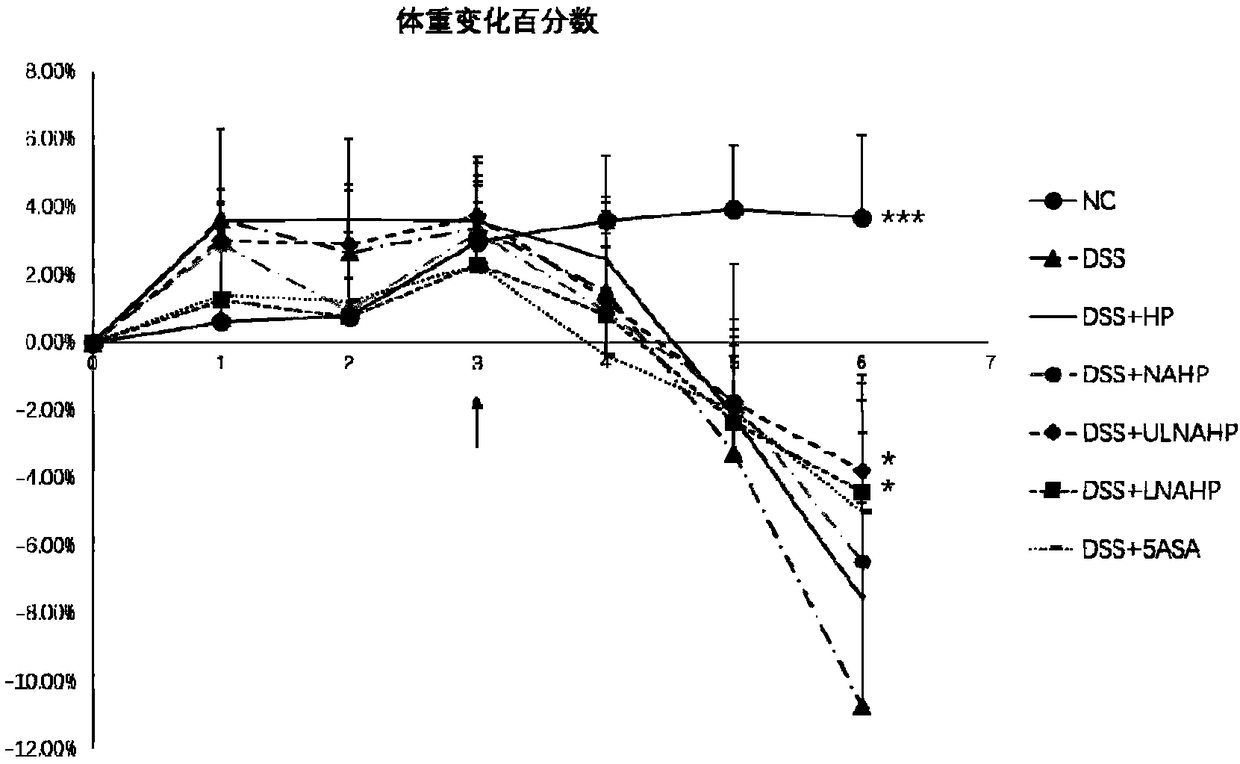
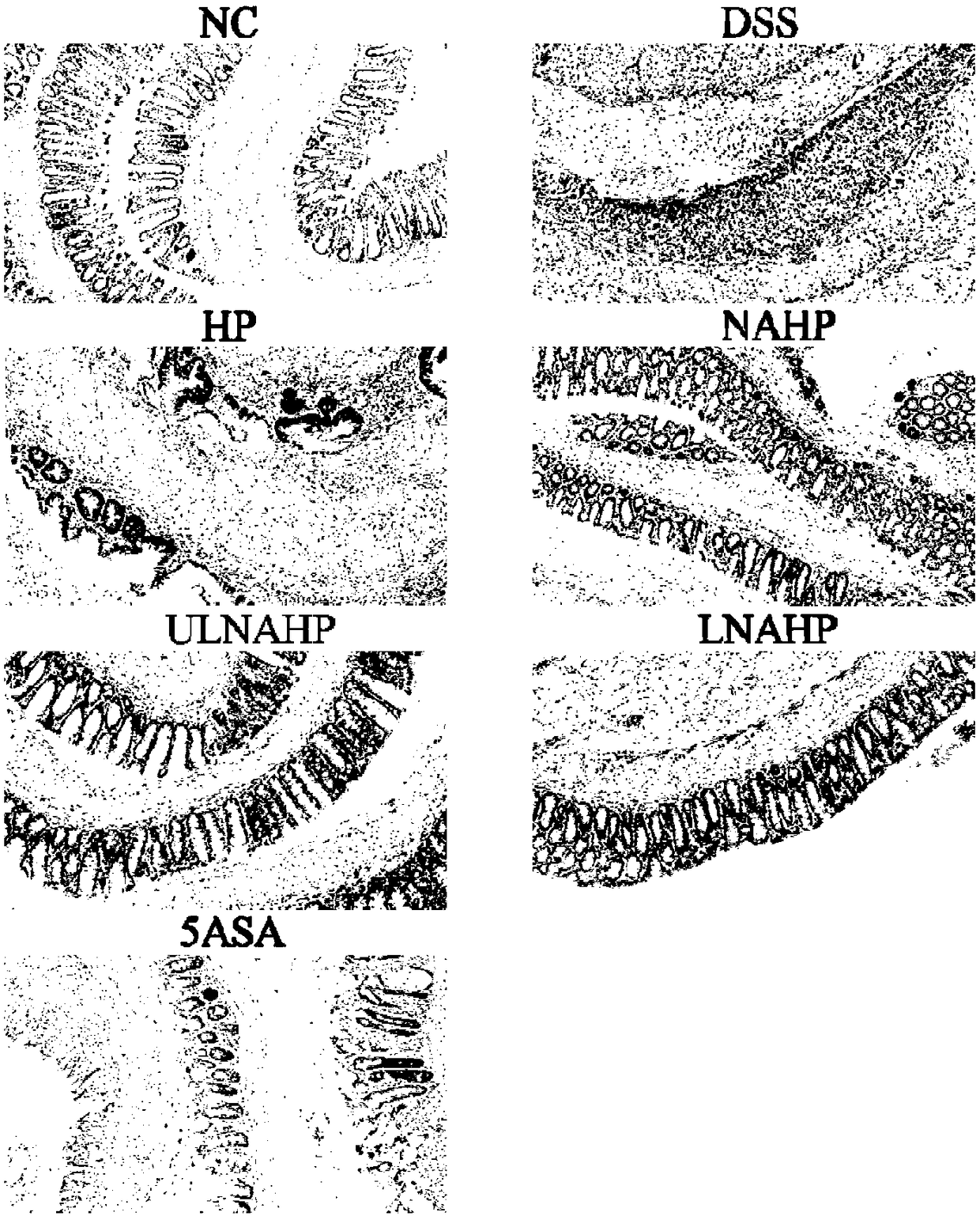
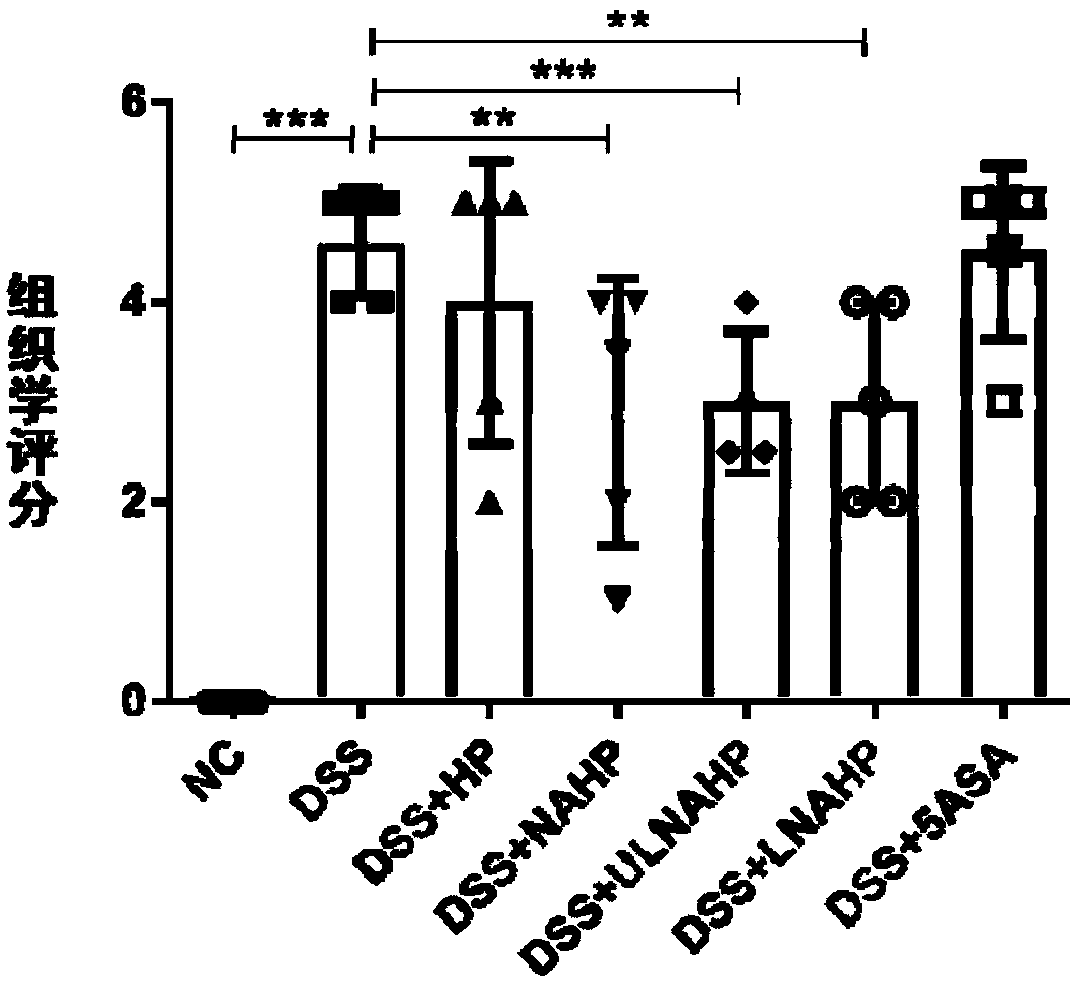
Anticoagulation-resistant heparin derivative
A technology of heparin derivatives and products, applied in anti-inflammatory agents, drug combinations, digestive system, etc., can solve problems such as disappointing experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Dissolve 20g of high-quality heparin (purchased from Changshan Biochemical Pharmaceutical Co., Ltd., product name: heparin sodium) in 0.6L deionized water, and add an equal volume of 0.2M sodium periodate to 0.6L of high-quality heparin (33g / L) Solution (prepared now), react at 300 rpm, 4°C and protect from light for 22 hours. Add 80mL of ethylene glycol to neutralize excess sodium periodate, then add 28g of sodium borohydride and react at 4°C for 16 hours. The pH was adjusted to 7.0 with HCl. Filter through a 0.22 μm filter membrane to collect filtered samples. Then use a dialysis bag to desalinate or use a Millipore ultrafiltration device with a 1K filter membrane for ultrafiltration concentration and desalination until the filtrate is passed through 0.1M AgNO 3 Desalination is considered complete when no color change is detected. The samples were frozen at -80°C and then placed in a lyophilizer to freeze-dry, and then crushed into powder with a mortar or a small p...
Embodiment 2
[0108] Dissolve the anticoagulant heparin prepared in Example 1 in the reaction buffer, add heparinase I prepared according to the method of ZL200410038098.6 to the solution every 0.5 to 1 h, add 20 IU heparinase I each time, and use the optical path difference Use a 1cm quartz cuvette and an ultraviolet spectrophotometer to monitor the light absorption A231 of the solution at 231nm (use a pH7.4 buffer to calibrate and zero the instrument. For the accuracy of the test results, when the ultraviolet spectrophotometer reading A231 is greater than 0.6 , dilute the solution by a certain number of times, so that the reading is measured at 0.2-0.6). When it is detected that A231 reaches 46, the reaction is terminated, and the enzyme activity of the added total heparanase I reaches about 220-250IU. The final method is to inactivate the enzyme in the reaction solution in a boiling water bath at 100°C for 5-10 minutes, then take out the reaction system and cool it to room temperature, a...
Embodiment 3
[0110] Dissolve the anticoagulant heparin prepared in Example 1 in the reaction buffer, add heparinase I prepared according to the method of ZL200410038098.6 to the solution every 0.5 to 1 h, add 20 IU heparinase I each time, and use the optical path difference Use a 1cm quartz cuvette and an ultraviolet spectrophotometer to monitor the light absorption A231 of the solution at 231nm (use a pH7.4 buffer to calibrate and zero the instrument. For the accuracy of the test results, when the ultraviolet spectrophotometer reading A231 is greater than 0.6 , dilute the solution by a certain number of times, so that the reading is measured at 0.2-0.6). When it is detected that A231 reaches 106, the reaction is terminated, and the enzyme activity of the added total heparanase I reaches about 340-380 IU at this time. The final method is to inactivate the enzyme in the reaction solution in a boiling water bath at 100°C for 5-10 minutes, then take out the reaction system and cool it to room...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com