A method for separation and analysis of derivatized heparan sulfate disaccharides containing free amino groups
A technology of heparan sulfate and free amino groups, which is applied in the field of separation and analysis of heparan sulfate disaccharides, can solve the problems of decreased sensitivity of mass spectrometry, contamination of ion sources of mass spectrometry, etc., and achieves the expansion of instrument application range, reduction of instrument cost, and great practical value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
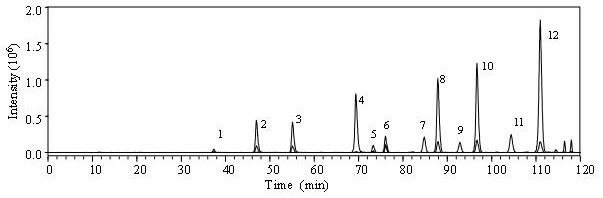
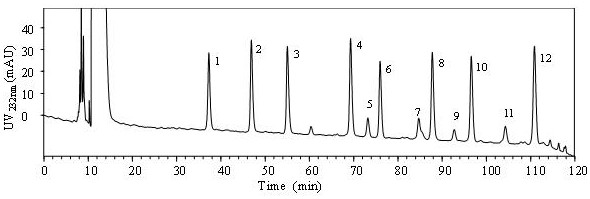
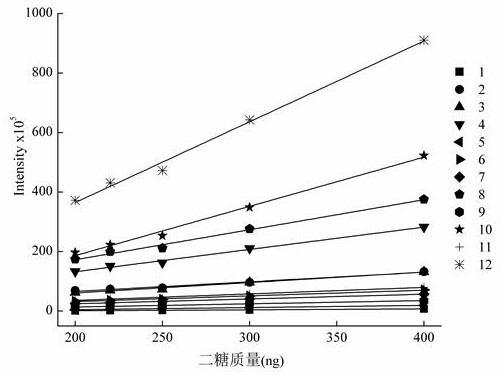
[0036]Use this technical solution to remove N-sulfated heparin (2S, 6S, NH) from self-made heparin derivatives3+-HP) and fully depleted 2,6,N-sulfated heparin (NH3+-HP) The disaccharide component of the sample is analyzed by an example.
[0037]A method for the separation and analysis of derivatized heparan sulfate disaccharides containing free amino groups. The steps are as follows:
[0038](1) Dissolve ammonium acetate in deionized water, adjust the pH to 5.6 with acetic acid, and prepare an ammonium acetate solution with a concentration of 40 mM, which is used as mobile phase A; methanol is used as mobile phase B.
[0039](3) Take 20μg each of the self-made heparin derivative de-N-sulfated heparin and fully de-N-sulfated heparin, and pass the mixture of heparinase Ⅰ, heparinase Ⅱ, and heparinase Ⅲ. After thorough enzymolysis, filter. Add 10 μL of 0.1 mol / L 2-aminoacridone solution (2-aminoacridone dissolved in a 3:17 acetic acid:dimethyl sulfoxide mixed solvent), and react for 20 minutes....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com