A production process for preparing anethole by catalyzing the dehydration of p-methoxyphenylpropanol
A technology of methoxyphenylpropanol and p-methoxybenzene, which is applied in the production process field of catalyzing the dehydration of p-methoxyphenylpropanol to prepare anethole, can solve the problems of complex post-processing, environmental pollution, etc., and achieve industrialization production, reduce the amount of three wastes, and reduce the effect of polymerization impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
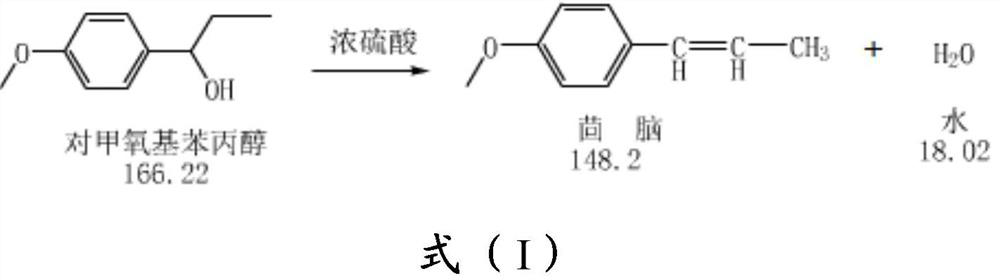
[0085] 3 g of acidic alumina and 100 g of methoxyphenol were put into four flasks, and then the reaction liquid was warmed to 120 ° C, and the water separator was separated until anhydrous fraction stopped.
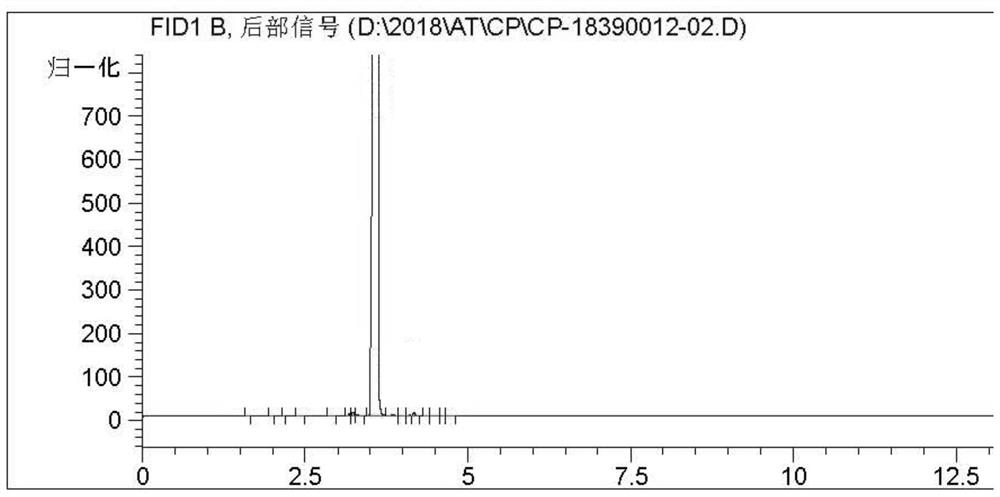
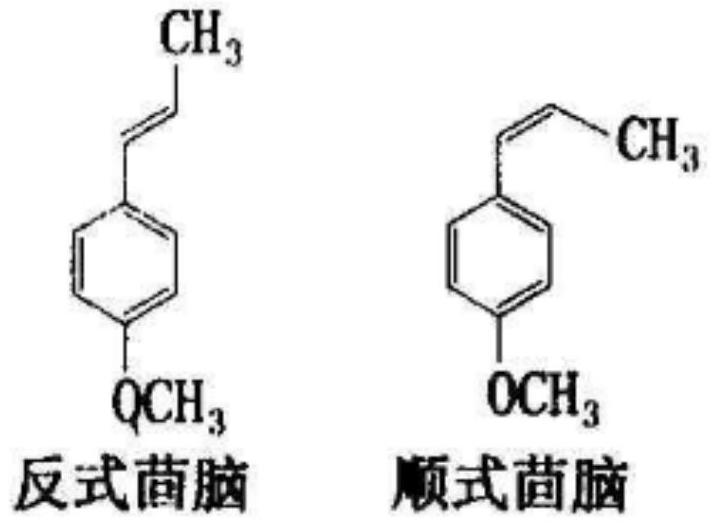
[0086] The reaction liquid Gc detection content, the cis is 6.3%, and the inverse fennel has a fluid content of 91.6%, the polymer content is 1.3%, and the selectivity of the fennel reaches 97.9%. The gas chromatogram of after separation of transfennel figure 1 Indicated. The product structure was analyzed by 1H-NMR analysis. Features: benzene ring resonance peaks (7.1-7.6 ppm, 4h); double bonds with a hydrogen resonance peak (6.5 ppm, 1h) of the benzene ring, double bonds with methyl Hydrogen resonance peak (6.1 ppm, 1H); methoxy carbon upper hydrogen resonance peak (3.8 ppm, 3h), and hydrogen resonance peaks (2.1 ppm, 3h) of the double-bonded methyl group.
Embodiment 2
[0088] 3 g of aluminum hydroxide and 100 g of methoxyphenol were put into four flasks, and then the reaction liquid was warmed to 120 ° C, and the water separator was separated until anhydrous fraction stopped.
[0089] The reaction liquid Gc was detected, the cis is 6.5%, and the inverse fennel has a fluid content of 90.3%, the polymer content is 2.3%, and the selectivity of the fennel has reached 96.8%.
Embodiment 3
[0091] The reaction conditions were the same as in Example 1, and the difference is only that the reaction temperature is 100 ° C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com