Amide Boc de-protection method
A kind of amide, selected technology, applied in the field of organic synthesis, can solve the problems of not being widely used, unfriendly to the environment, toxicity and corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
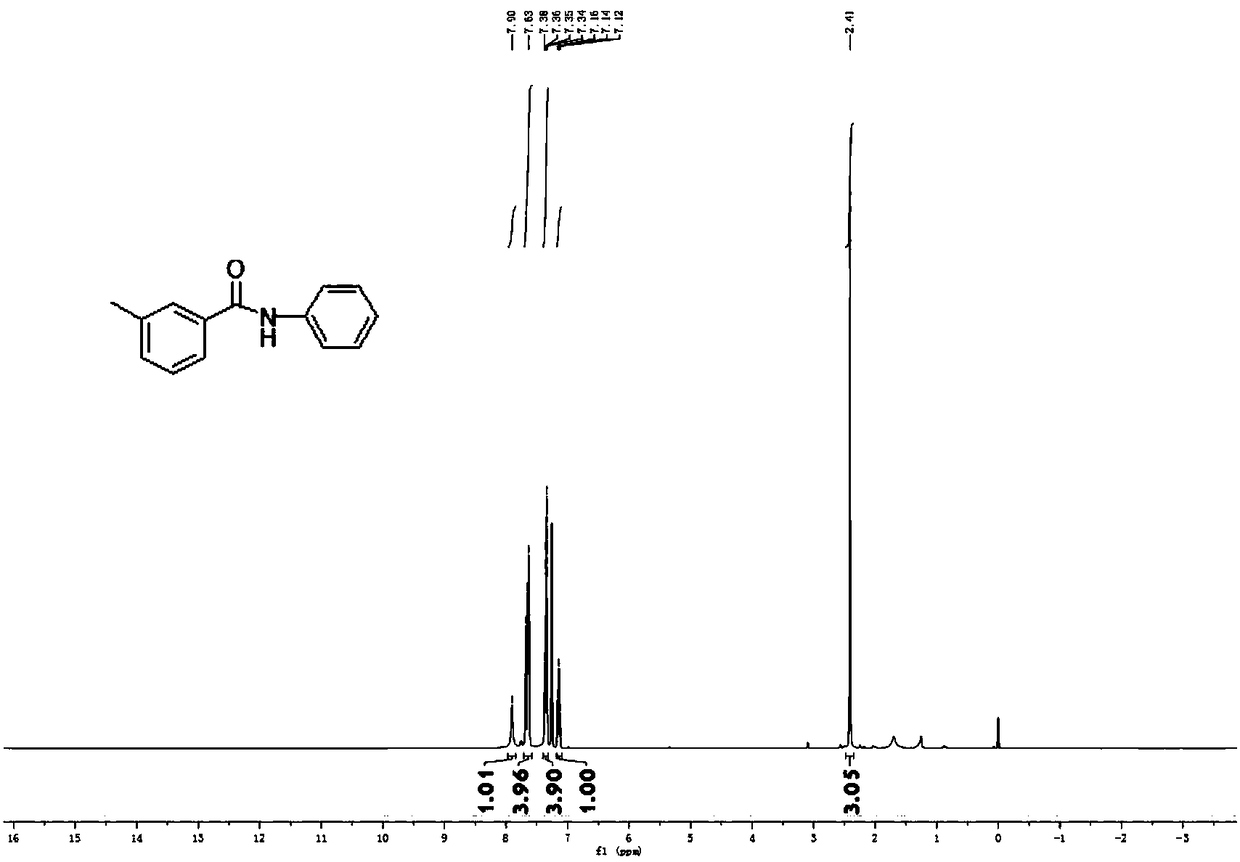
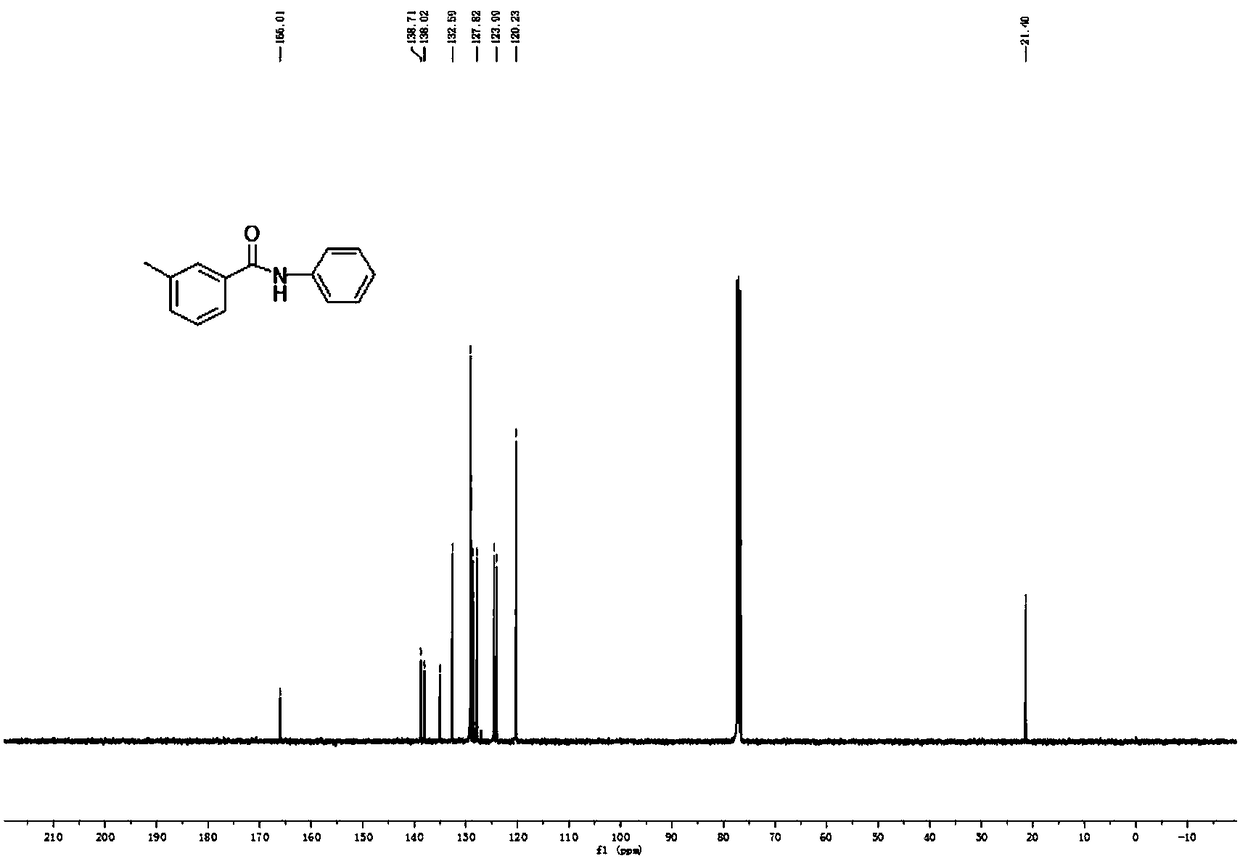
[0088] Embodiment 1, 3-methyl-N-phenylbenzamide
[0089]
[0090]
[0091] Weigh 0.5mmol amide III-1, 1% molar amount of palladium catalyst, 1mmol potassium carbonate in the Schlenk reaction tube, feed nitrogen into the double row tube, repeat three times, then open the large nitrogen valve, inject 0.75mmol aniline, add 2ml new Evaporate tetrahydrofuran and close the valve. The test tube was stirred at room temperature for 10 minutes and then placed at 110°C for 24 hours. After the reaction, spot the plate with TLC, spin dry tetrahydrofuran, add water, extract repeatedly three times with ethyl acetate, then dry with anhydrous magnesium sulfate, spin dry, and get 0.495 mmol (104.5 mg) of white crystals by column chromatography, which is the product 3-Methyl-N-phenylbenzamide, yield 99%.
[0092] 1 H NMR (400MHz, CDCl 3 )δ7.90(s, 1H), 7.71-7.58(m, 4H), 7.35(dd, J=8.8, 6.7Hz, 4H), 7.14(t, J=7.4Hz, 1H), 2.41(s, 3H ). 13 C NMR (101MHz, CDCl 3 )δ166.01, 138.71, 138.02, ...
Embodiment 2
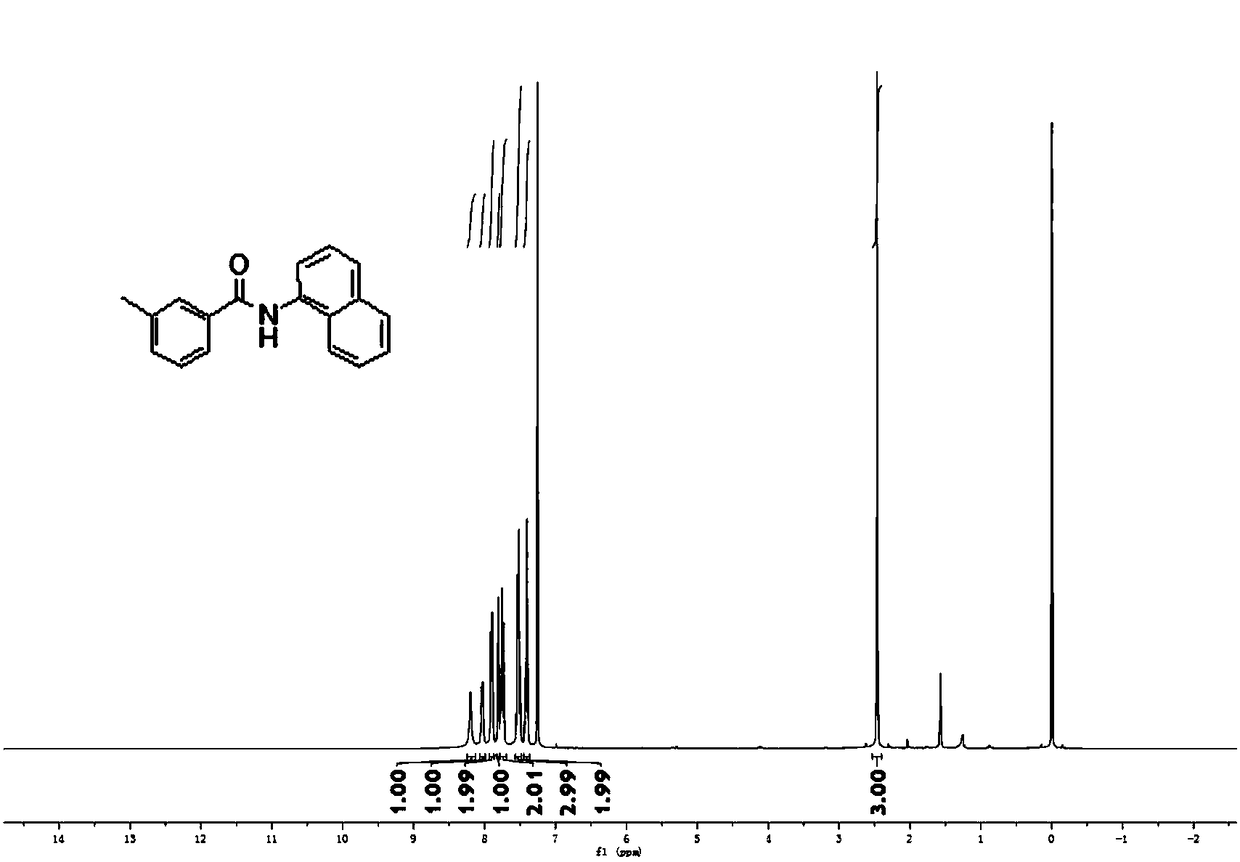
[0093] Embodiment 2, 3-methyl-N-naphthalene-1-yl-benzamide
[0094]
[0095] Weigh 0.5mmol amide III-1, 1% molar palladium catalyst, 1mmol potassium carbonate, 0.75mmol 1-naphthylamine in the Schlenk reaction tube, feed nitrogen into the double-row tube, repeat three times, then open the large nitrogen valve, add fresh steam 2ml tetrahydrofuran, close the valve. The test tube was stirred at room temperature for 10 minutes and then placed at 110°C for 24 hours. After the reaction was completed, TLC spotting, spin-dried tetrahydrofuran, added water, repeated extraction three times with ethyl acetate, then dried with anhydrous magnesium sulfate, spin-dried, and column chromatography obtained 0.425 mmol (111.8 mg) of white crystals of the product, namely Product 3-methyl-N-naphthalen-1-yl-benzamide, yield 85%.
[0096] 1H NMR (400MHz, CDCl3) δ8.20(s, 1H), 8.03(d, J=7.1Hz, 1H), 7.93-7.87(m, 2H), 7.81(s, 1H), 7.75(t, J= 7.6Hz, 2H), 7.57-7.48(m, 3H), 7.45-7.36(m, 2H), 2.46(s, 3...
Embodiment 3
[0097] Embodiment 3, 3-phenyl-N-(2-methylphenyl) benzamide
[0098]
[0099] Weigh 0.5mmol amide III-1, 1% molar amount of palladium catalyst, 1mmol potassium carbonate, 0.75mmol 2-methylaniline in the Schlenk reaction tube, feed nitrogen into the double row pipe, repeat three times, then open the nitrogen valve, add Freshly steam 2ml of tetrahydrofuran and close the valve. The test tube was stirred at room temperature for 10 minutes and then placed at 110°C for 24 hours. After the reaction, TLC plate, spin dry THF, add water, repeat extraction three times with ethyl acetate, then dry with anhydrous magnesium sulfate, spin dry, column chromatography to obtain the product white crystal 0.48mmol (108.0mg), namely Product 3-phenyl-N-(2-methylphenyl)benzamide, yield 96%.
[0100] 1 H NMR (400MHz, CDCl 3 )δ7.93(d, J=7.9Hz, 1H), 7.71(s, 1H), 7.65(d, J=4.1Hz, 2H), 7.42-7.33(m, 2H), 7.24(t, J=11.4 Hz, 2H), 7.12(d, J=7.4Hz, 1H), 2.44(s, 3H), 2.33(s, 3H). 13 C NMR (101MHz, CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com