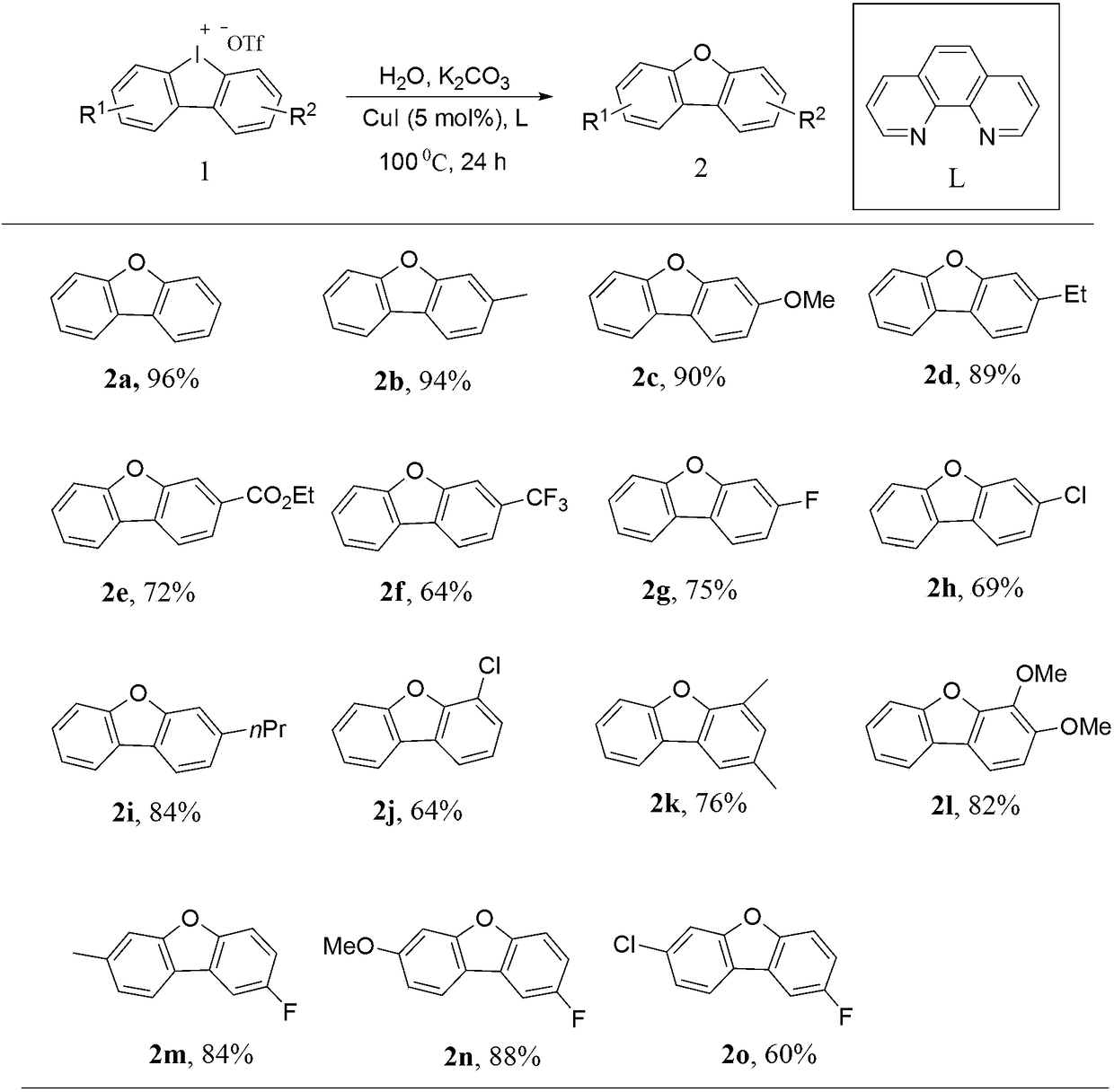
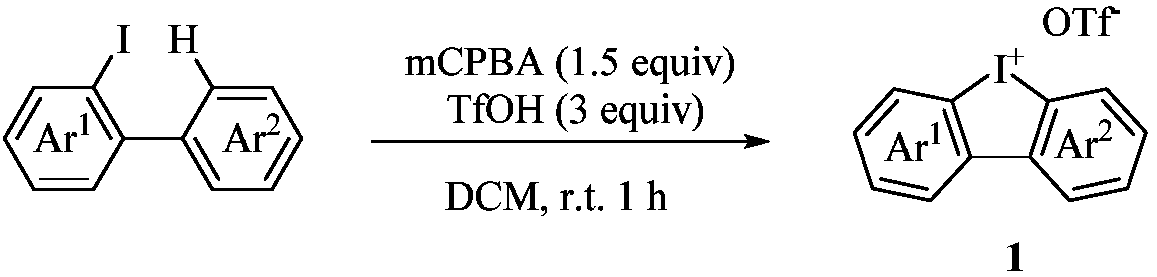Synthesis process of dibenzofuran derivatives
A technology of dibenzofuran and derivatives, which is applied in the fields of drug synthesis and material chemistry, can solve problems such as harsh reaction conditions, and achieve the effect of easy reaction and good universality of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] (1). Preparation of cyclic diaryliodonium salt 1:
[0016] Under ice bath, mCPBA (1.73 g, 7.5 mmol) was slowly added to a mixed solution of 2-iodobiaryl (5.0 mmol) and DCM (20 mL), stirred at room temperature for 40 minutes, and TfOH (1.32 mL, 15.0 mmol). Stir at room temperature for 2 hours, then remove DCM by evaporation in vacuo. Ether (15 mL) was added to the solid residue, stirred for 20 minutes, filtered, washed three times with ether (about 5 mL) and dried in vacuo to give the cyclic diaryliodonium triflate.
[0017]
Embodiment 1
[0019] Dibenzoiodopentacyclic trifluoromethanesulfonate (1.0mmol), 1,10-phenanthroline (0.1mmol), cuprous iodide (5mmol%), K 2 CO 3 (0.2mmol) and 2mL of water were added into a 15mL pressure-resistant tube, stirred at 100°C, and reacted for 24 hours. The yield of 2a obtained by petroleum ether recrystallization was 96%. 1 H NMR (400MHz, CDCl 3 ) 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=7.7Hz, 2H), 7.60(d, J=8.2Hz, 2H), 7.48(t, J=7.6Hz, 2H), 7.37(t, J=7.5Hz, 2H) .
Embodiment 2
[0021] 3-Methyl-dibenzoiodopentacyclic trifluoromethanesulfonate (1.0mmol), 1,10-phenanthroline (0.1mmol), cuprous iodide (5mmol%), K 2 CO 3 (0.2mmol) and 2mL of water were added to a 15mL pressure-resistant tube, stirred at 100°C, and reacted for 24 hours. The yield of 2b obtained by petroleum ether recrystallization was 94%. 1 H NMR (400MHz, CDCl 3 )δ7.95–7.91(m,1H),7.84(d,J=7.9Hz,1H),7.57(d,J=8.2Hz,1H),7.47–7.38(m,2H),7.34(td,J =7.5,0.8Hz,1H),7.19(d,J=7.8Hz,1H),2.55(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com