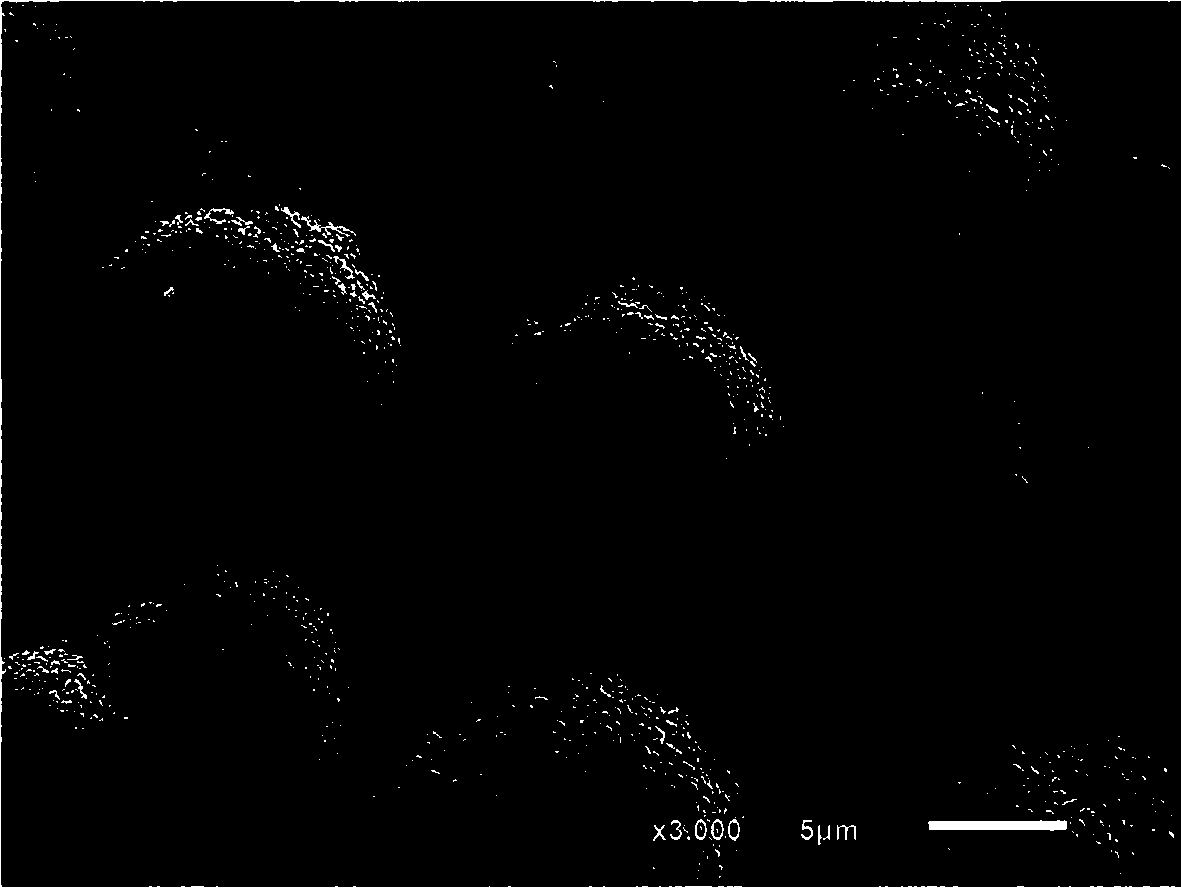
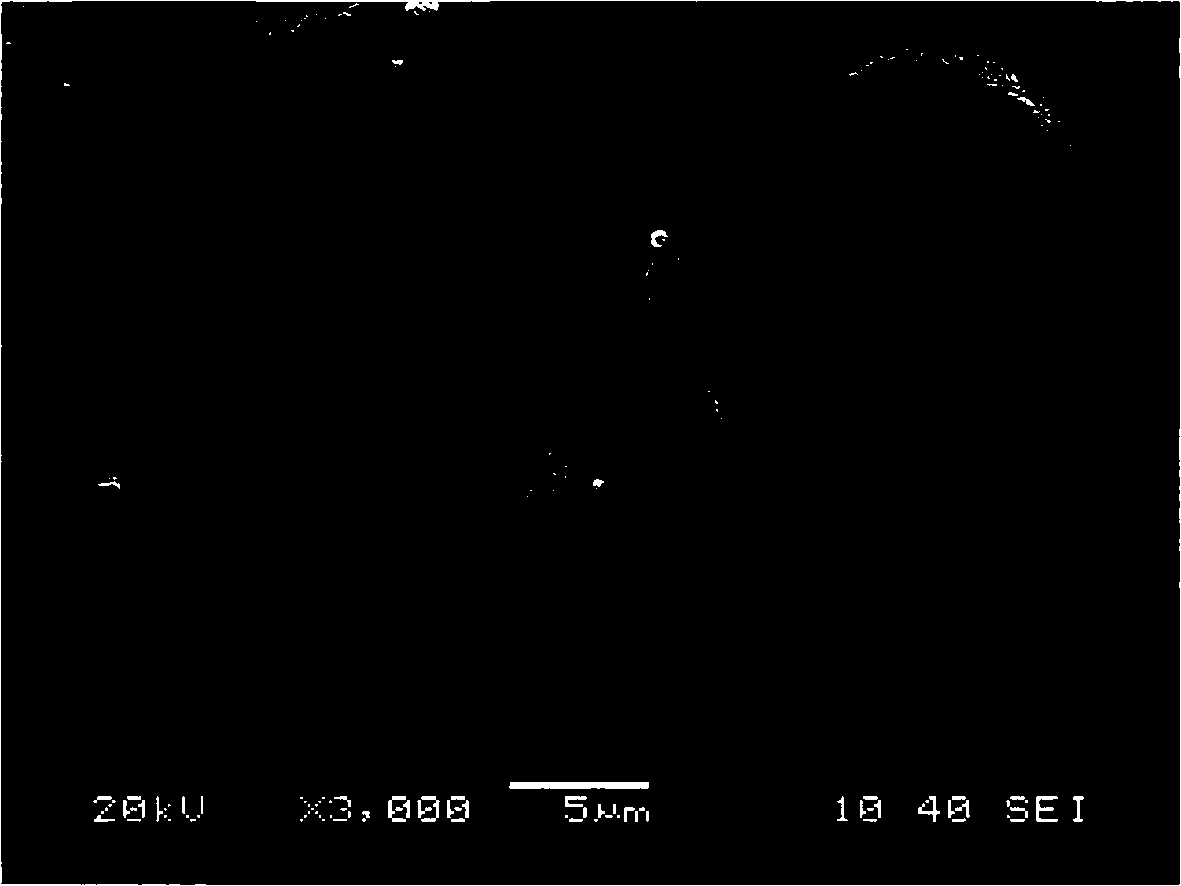
Preparation method of large-granularity cobaltosic oxide
A technology of tricobalt tetroxide and large particle size, which is applied in the direction of cobalt oxide/cobalt hydroxide, etc., can solve the problems of unfavorable industrial production and cumbersome process, and achieve the effect of avoiding excessive impurity content and simple requirements for calcination equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Prepare 1mol / L cobalt chloride solution as A solution, 1mol / L sodium carbonate solution as B solution, add 5 mL of hydrazine hydrate solution with a mass content of 30% for every liter of 5mol / L ammonia solution as C solution, 5mol / L The hydrogen peroxide solution is used as the D solution, and the 2mol / L sodium hydroxide solution is used as the E solution.
[0040] Synthesis reaction: at the beginning of the synthesis reaction, A solution, B solution, and C solution were added to the reaction kettle in parallel, and the cobalt carbonate synthesis reaction was carried out under strong stirring. During the reaction, the ammonia concentration of the reaction solution was strictly controlled to be 5g / L; The temperature of the system is 70°C, the flow rate of solution A is 100L / h for 0~1h, 200L / h for 1~2h, 300L / h for 2~3h, 400L / h for 3~4h, and 400L / h for 4h to synthesis The flow rate of the end time period is 500L / h, the flow rate of solution B is 1.02 times of the correspo...
Embodiment 2
[0047]Prepare 1.5mol / L cobalt sulfate solution as A solution, 1.5mol / L sodium carbonate solution as B solution, add 15mL hydrazine hydrate solution with a mass content of 30% for every liter of 8mol / L ammonia solution as C solution, 6mol / L The hydrogen peroxide solution is used as the D solution, and the 4mol / L sodium hydroxide solution is used as the E solution.
[0048] Synthesis reaction: at the beginning of the synthesis reaction, A solution, B solution, and C solution were added to the reactor in parallel, and the cobalt carbonate synthesis reaction was carried out under strong stirring. During the reaction, the ammonia concentration of the reaction solution was strictly controlled to be 8g / L; The temperature of the system is 65°C, the flow rate of solution A is 100L / h for 0~1h, 200L / h for 1~2h, 300L / h for 2~3h, 400L / h for 3~4h, and 400L / h for 4h to synthesis The flow rate of the end time period is 500L / h, the flow rate of solution B is 1.05 times of the corresponding flo...
Embodiment 3
[0055] Prepare 2mol / L cobalt nitrate solution as A solution, 2mol / L sodium carbonate solution as B solution, add 20mL hydrazine hydrate solution with a mass content of 30% for every liter of 10mol / L ammonia solution as C solution, and 8mol / L hydrogen peroxide Solution as D solution, 6mol / L sodium hydroxide solution as E solution.
[0056] Synthesis reaction: at the beginning of the synthesis reaction, A solution, B solution, and C solution were added to the reaction kettle in parallel, and the cobalt carbonate synthesis reaction was carried out under strong stirring. During the reaction, the ammonia concentration of the reaction solution was strictly controlled to be 6g / L; The temperature of the system is 75°C, the flow rate of solution A is 100L / h for 0~1h, 200L / h for 1~2h, 300L / h for 2~3h, 400L / h for 3~4h, and 400L / h for 4h to synthesis The flow rate of the end time period is 500L / h, the flow rate of solution B is 1.1 times of the corresponding flow rate of each flow rate zo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com