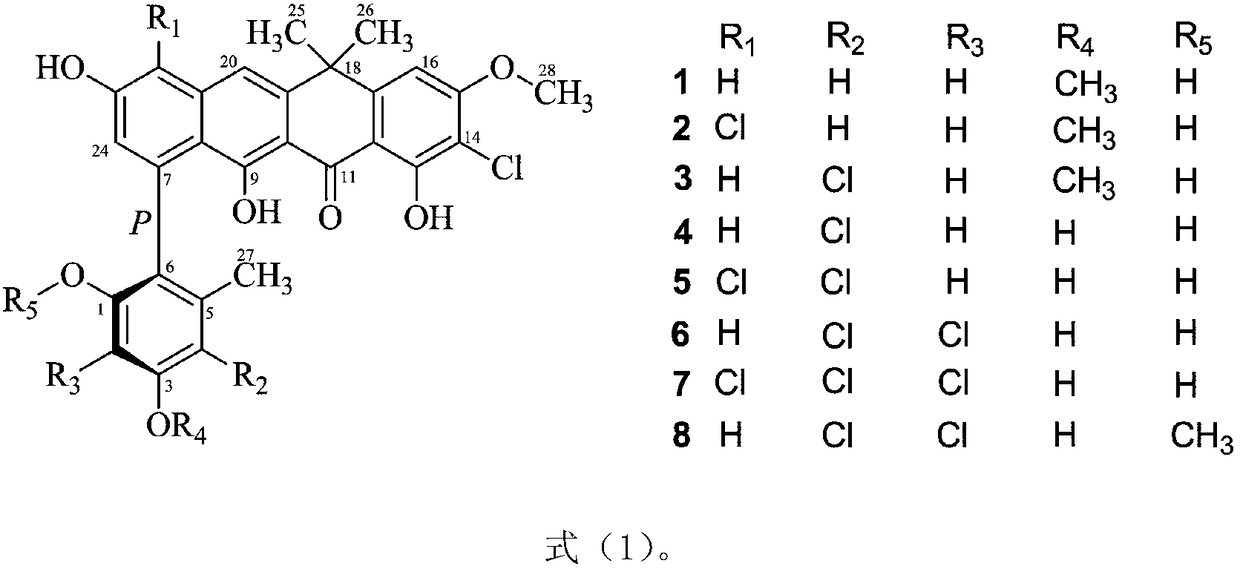
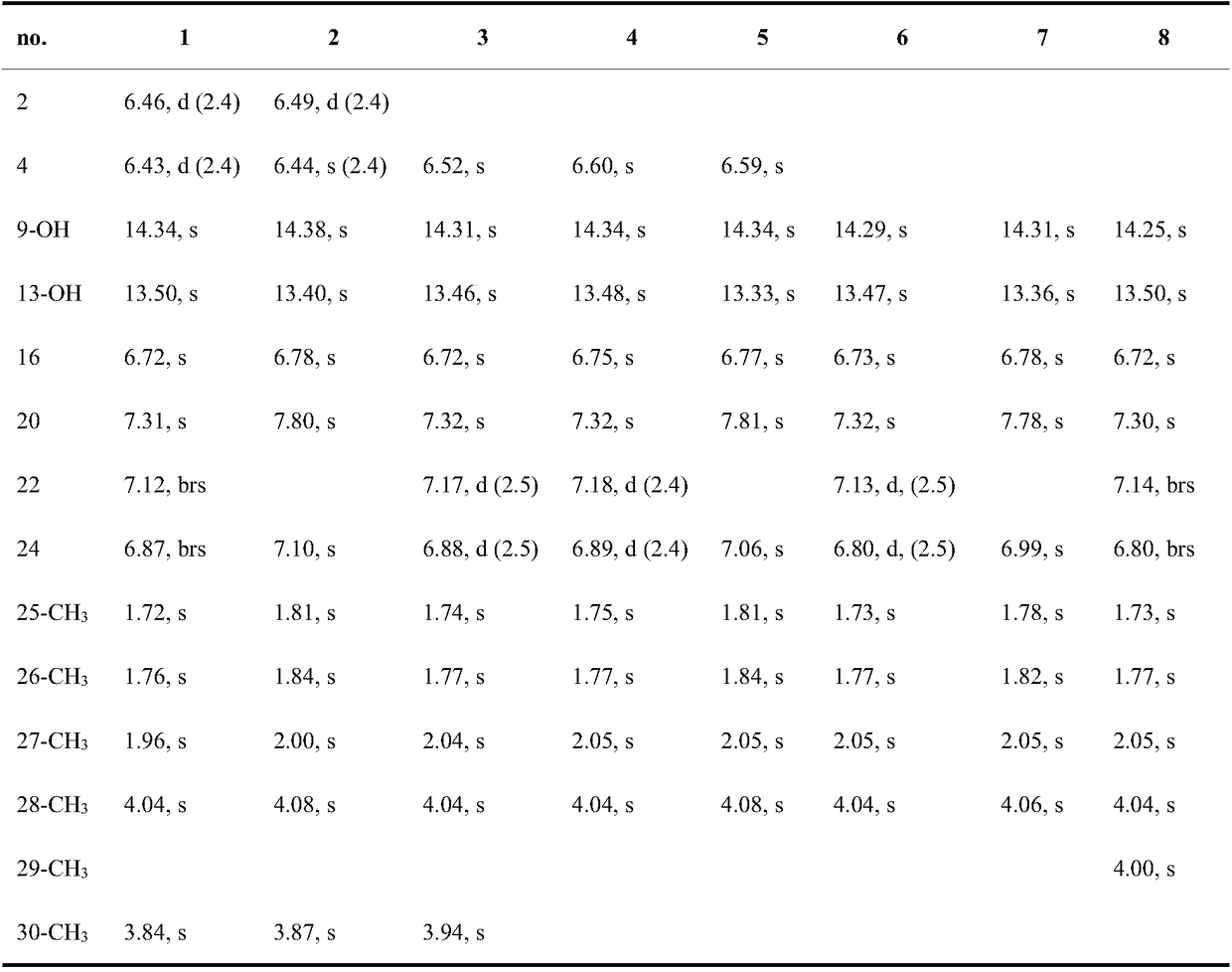
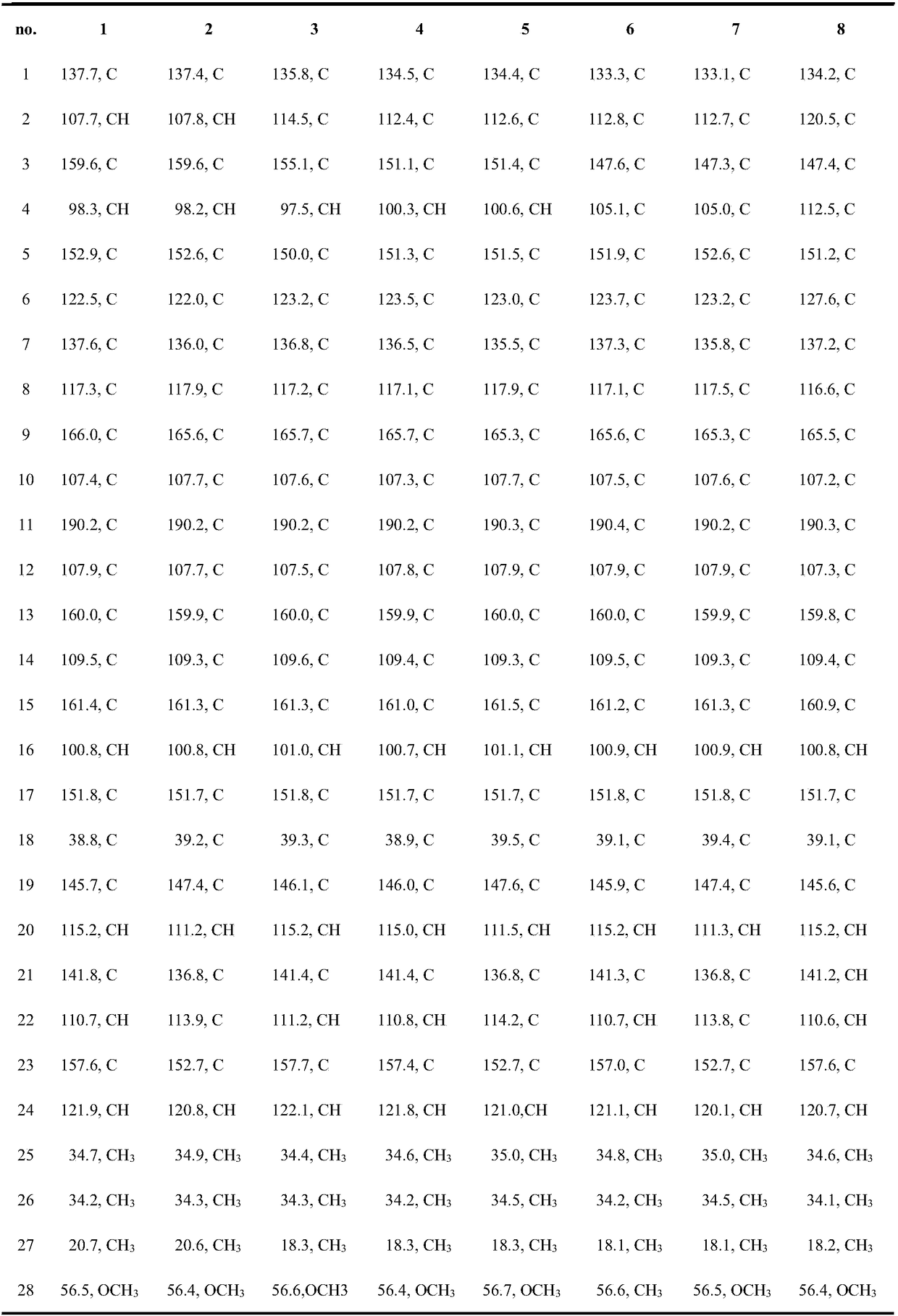
Fasamycins compounds, preparation method thereof and application thereof to preparation of antibacterial medicines
A technology of antibacterial drugs and compounds, applied in the field of biomedicine, can solve problems such as drug resistance, and achieve the effect of remarkable antibacterial activity, novel structure and high research and development potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Streptoverticillium morookaense SC1169 strain was inoculated on PDA solid medium, and cultured at 28° C. in the dark for 10 days for activation. Get an appropriate amount of above-mentioned activated bacterial strains and inoculate them into YMG medium (the preparation method of the YMG medium is: dissolve 4g of glucose, 10g of malt extract, and 4g of yeast extract in 1L of water, and sterilize for subsequent use) , 28° C., 150 rpm, and cultivated under dark conditions for 48 hours to obtain seed liquid. Then the above seed liquid was inoculated on wheat solid medium (50 g of wheat was added to every 50 mL of YMG medium, mixed uniformly and then sterilized), and cultured statically at 28° C. in the dark for 30 days to obtain a solid fermentation culture.
[0026]The solid fermentation culture was leached three times with 95% ethanol by volume fraction, each time for 48 hours. The extract was concentrated under reduced pressure to obtain the extract, which was dissolved...
Embodiment 2
[0043] Embodiment 2: Fasamycins compound antibacterial activity test shown in formula (1)
[0044]Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecium (VSE) and vancomycin-resistant Enterococcus faecium (VRE) were used as test strains, and 25 mL of MHB medium was used at 37 Cultivate on a shaker at 150 rpm for 12 h, adjust the concentration of each bacterial suspension to 1×10 with MHB 5 CFU / mL. Each test sample (compound 1-8) was dissolved in DMSO and diluted to the required concentration, the final concentration of each test sample was: 10, 5, 2.5, 1.25, 0.625 and 0.3125 μg / mL. Set the same concentration of DMSO instead of the test sample as a negative control, Alamar Blue suspension without bacteria as a blank control, and vancomycin as a positive control. Add 100 μL of bacterial suspension containing Alamar Blue (8%, v / v) and diluted test samples (4%, v / v) to a 96-well plate, and each treatment has three replicates, at 37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com