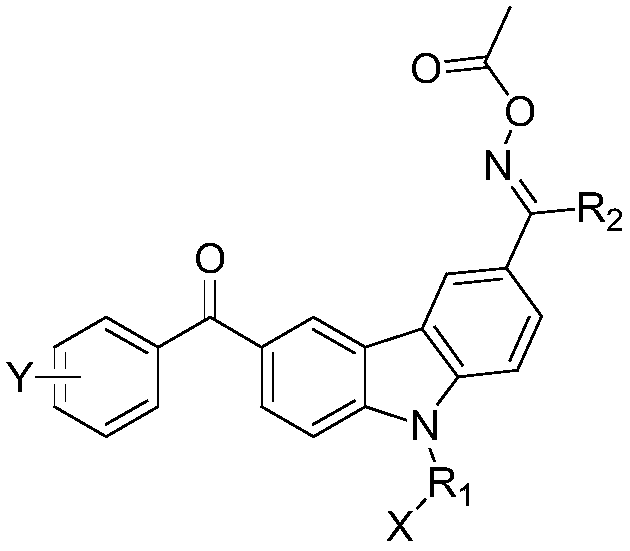
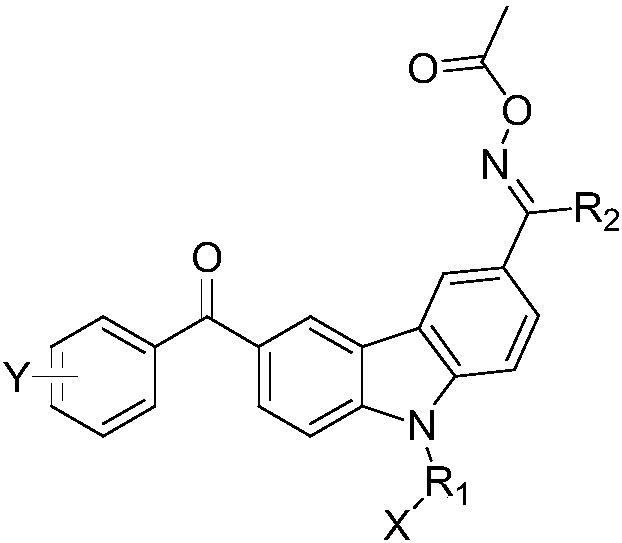
Oxime ester compound and a photocurable composition comprising the same
A photocurable, compound technology, applied in photosensitive materials, optics, organic chemistry, etc. for optomechanical equipment, which can solve problems such as reduction of foreign matter defect rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
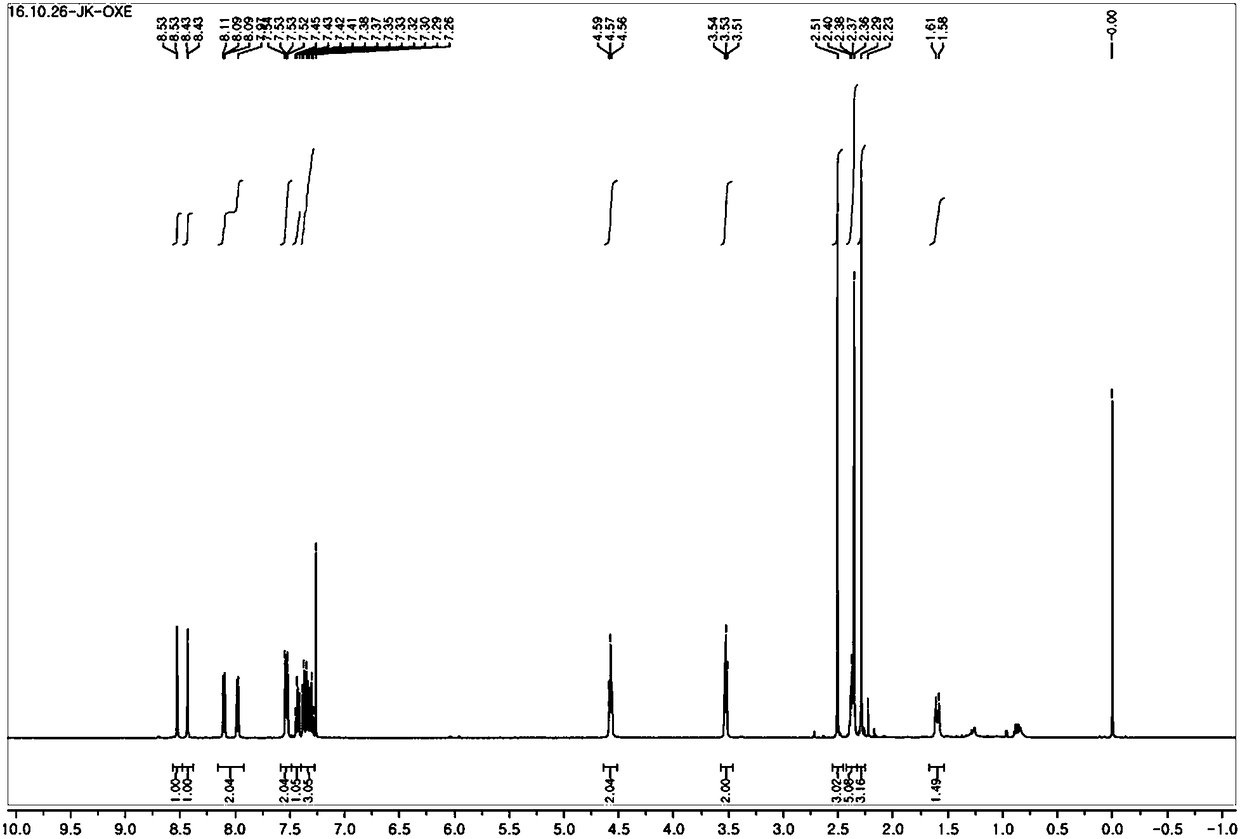
[0089] Production example 1. Production of oxime ester compound of chemical formula 2
[0090] [chemical formula 2]
[0091]
[0092] 1-1. Haloalkylation reaction of carbazole
[0093] [Reaction 1]
[0094]
[0095] After dissolving carbazole 25g (molecular weight 167.206; 0.15mol) in tetrahydrofuran 50mL and adding 1-bromo-3-chloropropane 70.6g (molecular weight 157.44; 0.45mol) and tetrabutylammonium bromide 0.36g solution, add 50% caustic soda 48g, then heated and stirred at 45-50°C for 5-6 hours.
[0096] After the reaction liquid was cooled to normal temperature and the upper layer was separated, the tetrahydrofuran was removed by reducing pressure, and then the excess 1-bromo-3-chloropropane was removed to obtain 20 g of the compound of the following chemical formula 3 in the oil phase (molecular weight: 243.73100; 0.083mol).
[0097] [chemical formula 3]
[0098]
[0099] 1-2. Acylation reaction of the compound of chemical formula 3
[0100] Dissolv...
manufacture example 2
[0118] Production example 2. Production of oxime ester compound of chemical formula 6
[0119] [chemical formula 6]
[0120]
manufacture example 2-1
[0121] Production Example 2-1. Haloalkylation reaction of carbazole
[0122] In addition to using 1-bromo-3-fluoropropane (1-Bromo-3-fluoropropane) instead of 1-bromo-3-chloropropane to carry out the reaction, in the haloalkylation reaction with Production Example 1-1. Carbazole The reaction is carried out under the same conditions to obtain the compound of chemical formula 7.
[0123] [Reaction 5]
[0124]
[0125] [chemical formula 7]
[0126]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com