Asymmetric aluminum complex containing o-phenylenediamine group and its preparation method and application
An o-phenylenediamine-based, aluminum complex technology is applied in the field of asymmetric aluminum complexes, which can solve the problems of non-renewability and non-degradability, and achieve good molecular weight controllability, high stereoselectivity, and fast reaction rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
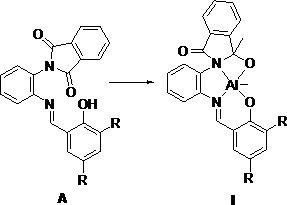
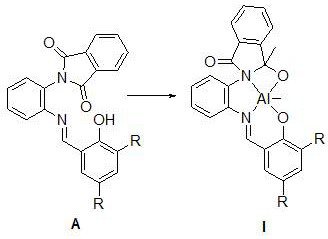
[0046] The structural formula of the synthesized ligand is the above formula (A), where R is hydrogen, and the reaction process is as follows: add 0.40 g of unilaterally protected o-phenylenediamine (a) and an equimolar amount of salicylaldehyde into 20 mL of methanol, and heat to reflux After 12 hours of reaction, after the reaction was completed, it was cooled and filtered, washed with cold methanol, filtered, collected, dried and weighed to obtain 0.50 g of solid, with a yield of 87.7%.
[0047] The product obtained is characterized, and the results are as follows:
[0048] 1 H NMR (400 MHz, CDCl 3 ) δ 12.86 (s, 1H, O H ), 8.42 (s, 1H, Ar H C=N), 7.85(m, 2H, Ar– H ), 7.64 (d, J = 7.0 Hz, 1H, Ar– H ), 7.56 (m, 1H, Ar– H ), 7.44 (m,3H, Ar– H ), 7.32 (m, 2H, Ar– H ), 6.96 (m, 4H, Ar– H ).
[0049] HRESI-MS: m / z cacld.C 21 h 14 N 2 o 3 [M-H] - ; 341.0926, found: 341.0924.
[0050] It can be seen from the above characterization results that the obtained produ...
Embodiment 2
[0052] The structural formula of the synthesized ligand is the above formula (A), where R is a methyl group, and the reaction process is as follows: 0.30 g of unilaterally protected o-phenylenediamine (a) and an equimolar amount of 3,5-dimethyl salicylaldehyde Add 20 mL of methanol, heat to reflux for 12 hours, cool and filter after the reaction, wash with cold methanol, filter, collect, dry and weigh to obtain 0.40 g of solid, with a yield of 85.1%.
[0053] The product obtained is characterized, and the results are as follows:
[0054] 1 H NMR (400 MHz, CDCl 3 ) δ 12.72 (s, 1H, O H ), 8.40 (s, 1H, Ar H C=N), 7.80(m, 2H, Ar– H ), 7.66 (m, 2H, Ar– H ), 7.15 (m, 3H, Ar– H ), 6.92 (s, 1H, Ar– H ),2.15 (s, 3H, ArC H 3 ), 2.04 (s, 3H, ArC H 3 ). HRESI-MS: m / z ccld. C 23 h 18 N 2 o 3 [M-H] - ; 369.1238, found: 369.1238.
[0055] It can be seen from the above characterization results that the obtained product is the ligand in which R is a methyl group in the above...
Embodiment 3
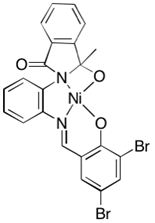
[0057] The structural formula of the synthesized ligand is the above formula (A), where R is bromine, and the reaction process is as follows: add 0.35 g of unilaterally protected o-phenylenediamine (a) and 3,5-dibromosalicylaldehyde in 20 In mL methanol, heated to reflux for 12 hours, after the reaction was completed, cooled and filtered and washed with cold methanol, filtered, collected, dried and weighed to obtain 0.66 g of solid, with a yield of 90.4%.
[0058] The product obtained is characterized, and the results are as follows:
[0059] 1 H NMR (400 MHz, CDCl 3 ) δ 12.64 (s, 1H, O H ), 8.344 (s, 1H, Ar H C=N),7.86 (d, J = 7.2 Hz, 1H, Ar– H ), 7.68 (m, 3H, Ar– H ), 7.42 (m, 2H, Ar– H ), 7.36(m, 1H, Ar– H ), 7.10 (m, 3H, Ar– H ).
[0060] HRESI-MS: m / z cacld.C 21 h 12 Br 2 N 2 o 3 [M-H] - ; 496.9134, found: 496.9136.
[0061] It can be seen from the above characterization results that the obtained product is the ligand in which R is bromine in the above f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com