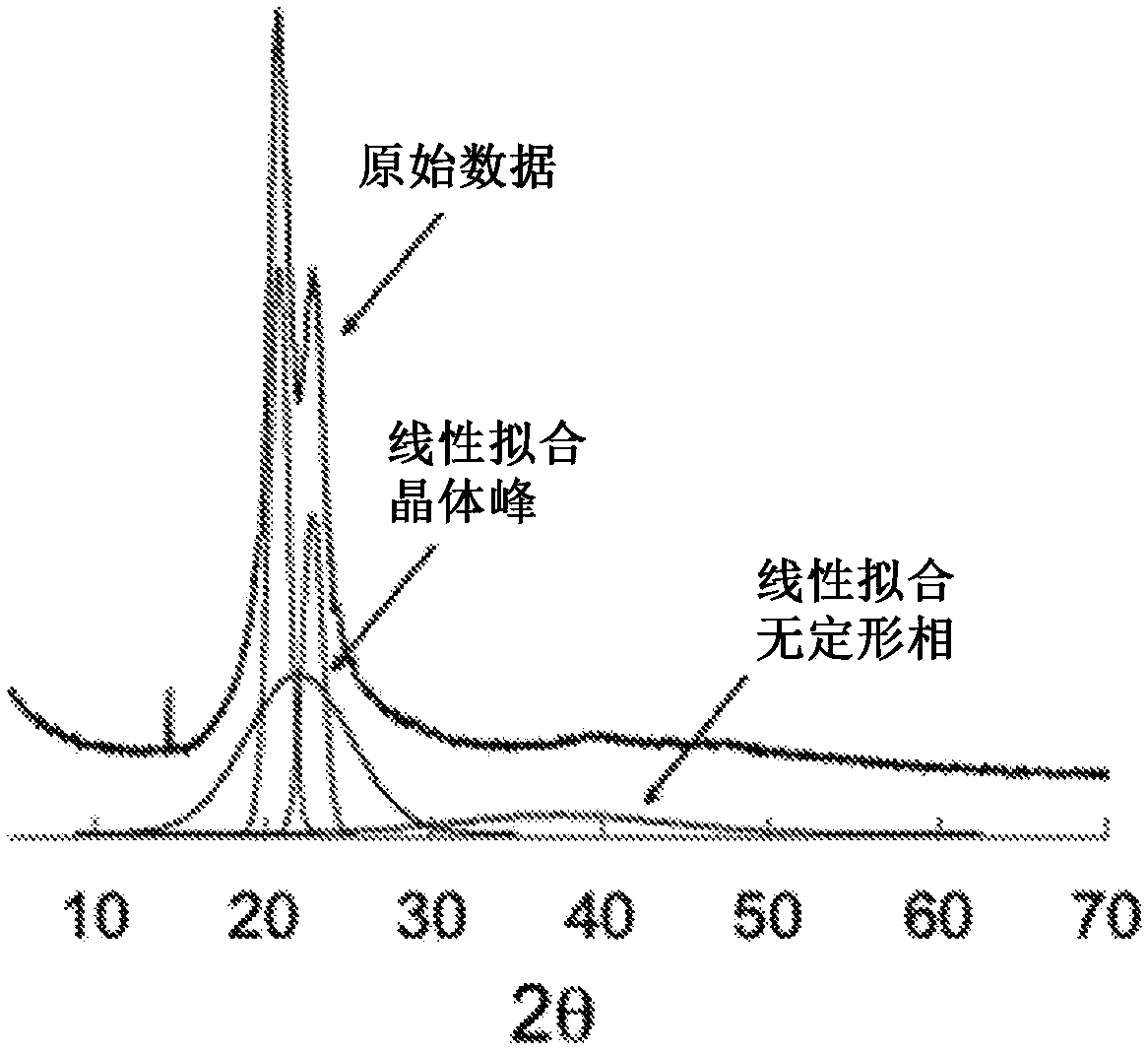
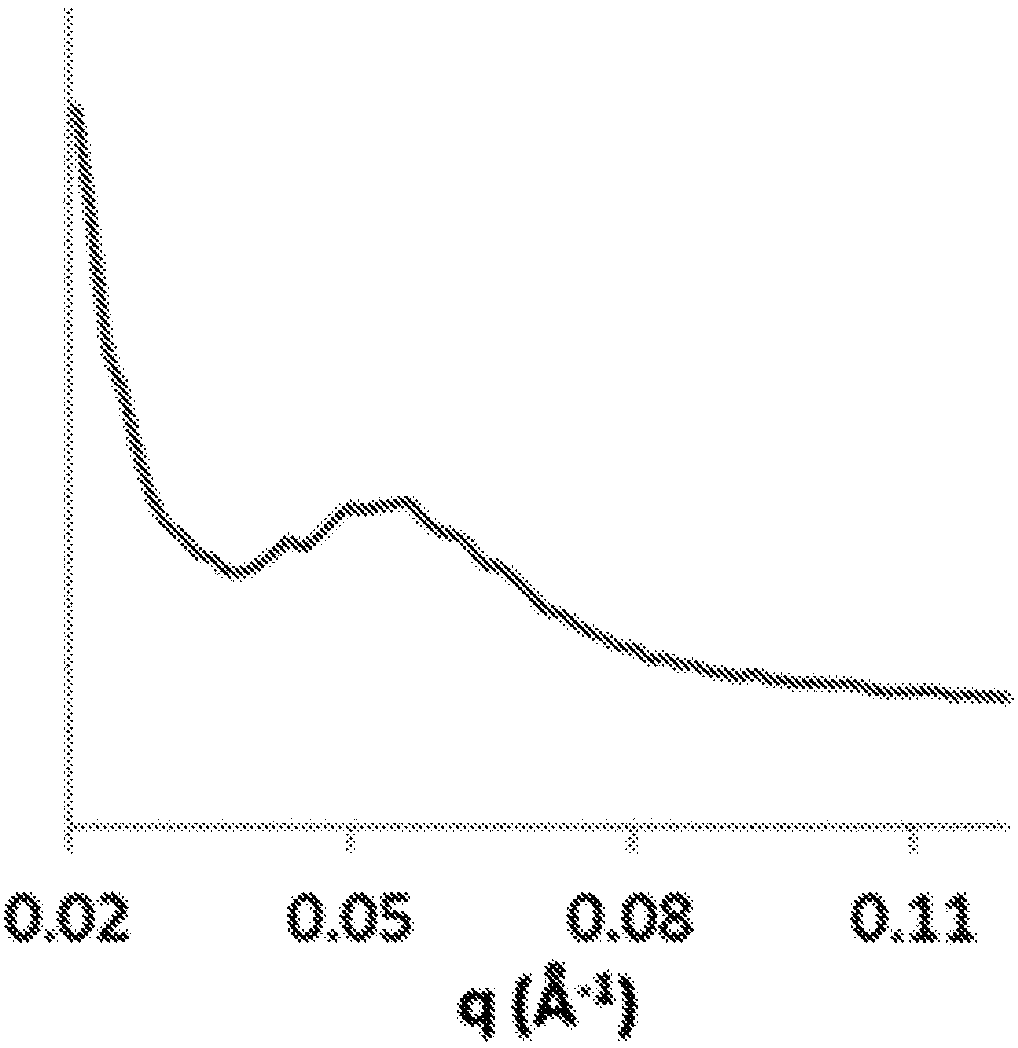
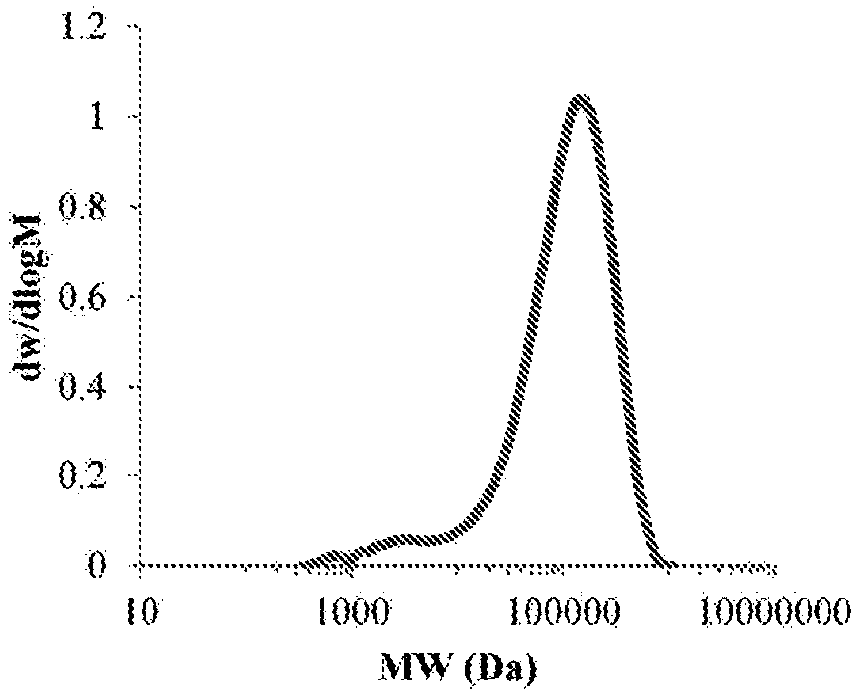
Biomimetic synthetic rubber and methods for controlling its physical properties through backbone double bond stereochemistry
一种组合物、单体的技术,应用在仿生弹性体组合物领域,能够解决官能团结合至材料、限制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] A. Synthetic steps
[0152] 1. Reagents
[0153] The following chemicals were used as received: acetonitrile (MeCN: Sigma-Aldrich, >99.5%), 1,4-butanediol (Sigma-Aldrich, 99%), chloroform (CHCl 3 : VWR Chemicals, 99%), d-chloroform (CDCl 3 : Apollo, >99%), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU: Sigma-Aldrich, 98%), diethyl ether (Et 2 O: Sigma-Aldrich, ≥99.8%), N,N-dimethylformamide (DMF: Fisher Scientific, LR grade), 2,6-di-tert-butyl-4-methylphenol (BHT: Alfa Aesar, 99%), ethyl acetate (EtOAc: Fisher Scientific, LR grade), hexane (VWR Chemicals, 99%), 1,6-hexanediol (Sigma-Aldrich, 99%), magnesium sulfate (MgSO 4 : Anhydrous, Fisher Scientific, LR grade), 1,3-propanediol (Sigma-Aldrich, 98%), propiolic acid (Acros Organics, 98%), silica gel (SiO 2 : Apollo Scientific, 40-63 microns), Sodium Chloride (NaCl: Fisher Scientific, >99%), Sodium Bicarbonate (NaHCO 3 : Fisher Scientific, >99%), sulfuric acid (Fisher Scientific, >95%), triethylamine (Et 3 N: Fisher S...
Embodiment 2
[0300] Dithiol monomer was obtained from commercial sources and used after purification by distillation. Multifunctional alkyne monomers were obtained by sulfuric acid-catalyzed Fischer esterification of propiolic acid and the corresponding diol in a 2:1 ratio using a Dean-Stark apparatus at 120 °C. At -15 °C, before adding 1 mol% 1,8-diazabicycloundecene (DBU) as a catalyst, by 3 The combination of diyne and dithiol monomers in equimolar ratios undergoes thiol-yne step-growth polymerization. The exothermic reaction was allowed to warm to room temperature and stirred for 1 h, then washed with CHCl 3 After dilution, 2,6-di-tert-butyl-4-methylphenol (BHT, 5%wt) was added to prevent undesired free radical reaction and precipitation into ether. The molar mass of the polymer was varied by varying the amount of dithiol relative to the diyne so that the diyne was always in excess, and by using Et 3 N instead of DBU as catalyst while changing solvent and CHCl 3 and DMF composition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight-average Molecular Weight | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| tensile modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com