Water-soluble pregnenolone derivative and use thereof
A technology of pregnenolone and derivatives, which is applied in the field of water-soluble pregnenolone derivatives and their uses, and can solve problems such as potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
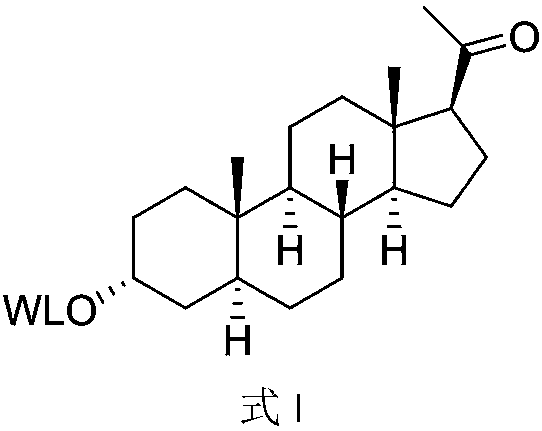
[0076] W is the preparation of the formula I compound of W-1
[0077] Dissolve N-tert-butoxycarbonyl-glycine (10mmol), allopregnenolone (5mmol), and DCC (15mmol) in dry dichloromethane (100ml), add DMAP (5%), react at room temperature for 24h, filter, The organic layer was washed successively with saturated aqueous ammonium chloride solution (40ml*3), saturated sodium bicarbonate (30ml*3) and saturated brine (40ml), the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was subjected to column chromatography Separation gives N-tert-butoxycarbonyl-glycine-pregnenolone ester.
[0078] Dissolve N-tert-butoxycarbonyl-glycine-pregnenolone ester in ethyl acetate, pass through dry HCl gas, react for 16 hours, concentrate, and crystallize the residue with ethanol and methyl tert-butyl ether to obtain W It is the compound of formula I of W-1.
Embodiment 2
[0080] W is the preparation of the formula I compound of W-5
[0081] With allopregnenolone and N-tert-butoxycarbonyl-valine as raw materials, according to the operation of Example 1, the compound of formula I with W as W-5 can be obtained.
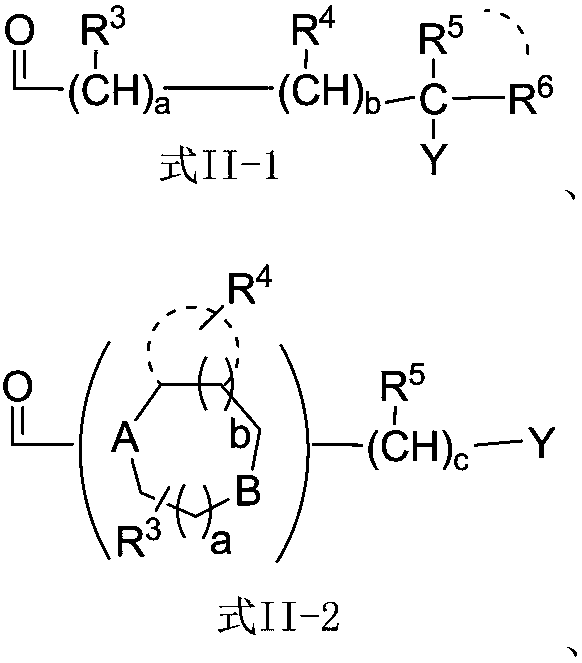
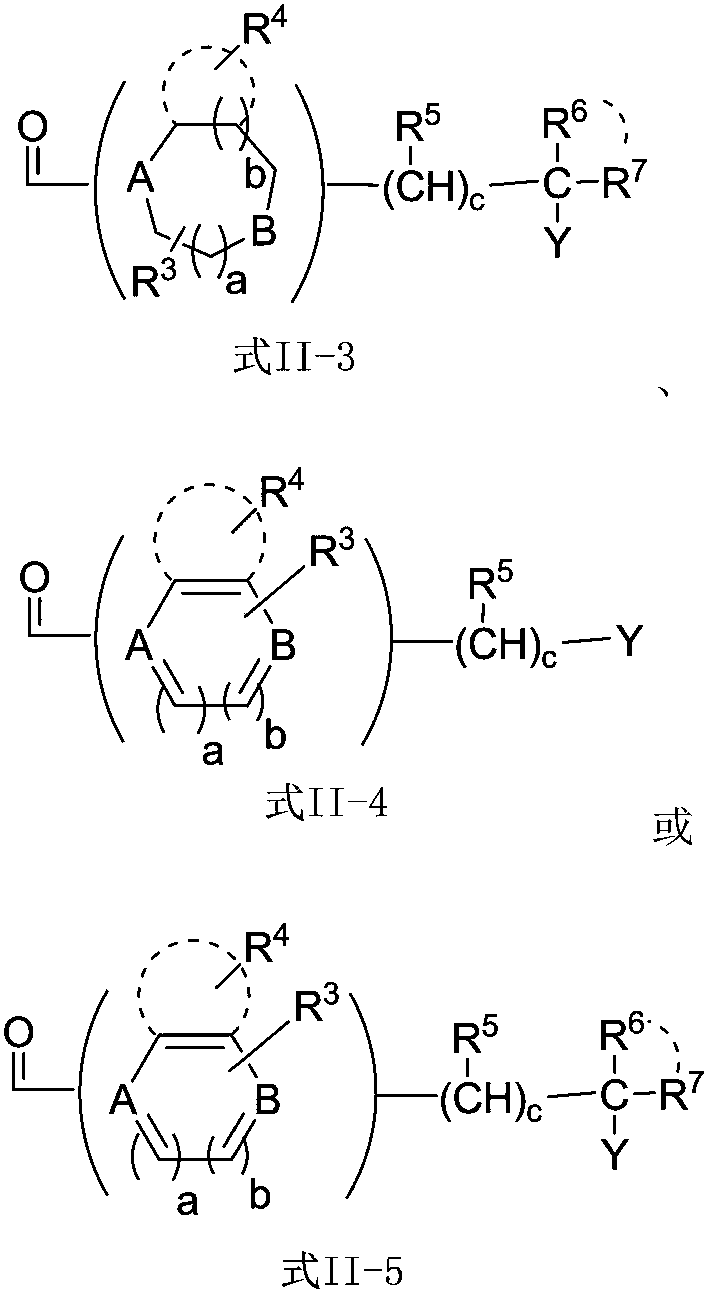
[0082] Using different protecting groups for different amino acids, according to the operation of Example 1, the compound of formula I in which W is W-(1-25) can be prepared.
Embodiment 3
[0084] W is the preparation of the formula I compound of W-27
[0085] Suspend 4-N,N-dimethylaminobutyric acid hydrochloride (10mmol) in dry DCM (40ml), add oxalyl chloride (100mmol) and a drop of DMF, stir overnight at room temperature, evaporate the solvent and excess Oxalyl chloride, the residue was dissolved in dry DCM (30ml) and set aside.
[0086] Dissolve allopregnenolone (5mmol) and DMAP (11mmol) in dry DCM (50ml), cool in an ice bath, slowly add the acid chloride prepared in the step above, after the addition, react at room temperature, detect by HPLC, and react After that, the organic layer was washed successively with saturated ammonium chloride (30ml*3) and saturated brine (30ml), the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was crystallized with ethanol and ether to obtain W as W-27 The compound of formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com