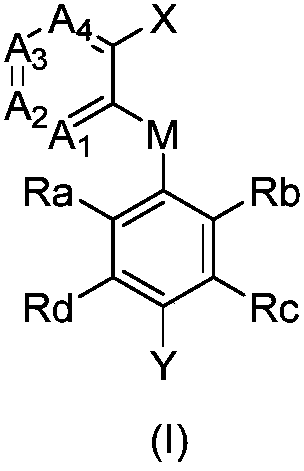
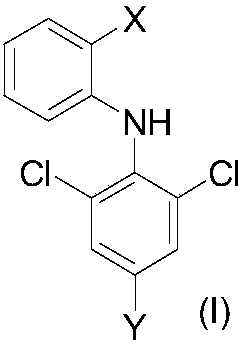
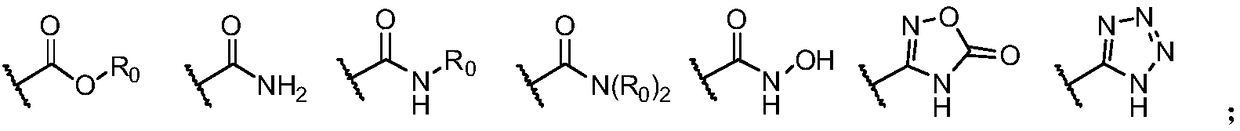
2-(substituted benzene matrix) aromatic formic acid FTO inhibitor, preparation method and application thereof
An unsubstituted, heterocyclic group technology, applied in the field of pharmaceutical compounds, can solve the problems of multiple deformities, growth retardation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Preparation of 2-(substituted phenylhelicoyl)aromatic formic acids compound 15 and compound 25
[0118] The synthesis of compound 25 starts with o-iodobenzoic acid and 4-bromo-2,6-dichloroaniline, Ullman coupling occurs under the action of anhydrous copper acetate and N-methylmorpholine, undergoes esterification, Suzuki coupling reaction, compound 15 can be obtained by hydrolysis, and compound 25 can be obtained by hydroxamation on this basis, as shown in the figure below:
[0119]
[0120] The first step: 30g (120mmol, 1.2eq) of o-iodobenzoic acid, 24g (100mmol, 1.0eq) of 2,6-dichloro-4-bromoaniline, triethylamine (150mmol, 1.5eq) and anhydrous copper acetate 9g (5.0mmol, 0.5eq) was dissolved in 500mL of DMF, heated to 120°C for 24h under the protection of argon, and the reaction was completed. Cool down to room temperature, add an equal volume of water, extract the mother liquor with DCM 300mL×3, wash the DMF with water, spin the organic phase to dry, pass the col...
Embodiment 2
[0282] The following is the effect of 2-(substituted phenylheteroyl) aromatic formic acid and its derivatives FTO inhibitors shown in general formula (I) on solid tumor human small cell lung cancer cell line (SCLC-21H), human bone marrow rhabdomyocarcinoma cell line (RH30) and pancreatic cancer cell line (KP3) cytotoxicity studies:
[0283] Culture SCLC-21H, RH30, KP3 and other solid tumor cell lines respectively, plant cells in a 96-well plate at a density of 5,000 per well, culture the cells until they adhere to the wall, add different compounds and continue to culture for 72 hours, and directly add 10uL of MTS solution for incubation 4h, the absorbance value at 490nm was detected, and the inhibition rate was calculated with the DMSO group as the control.
[0284] The following is the cytotoxicity of the 2-(substituted phenylhelicoyl) aromatic formic acid compound FTO inhibitor represented by the general formula (I) at a concentration of 50 μM and a time point of 72 hours to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com