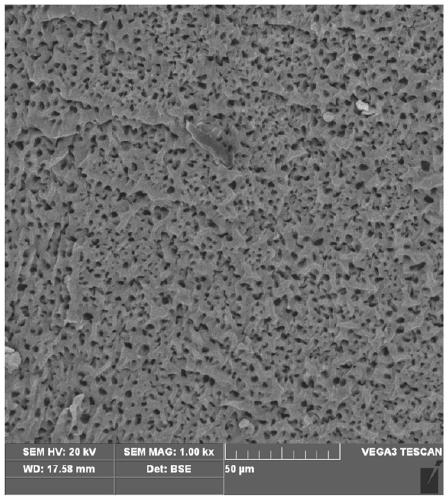
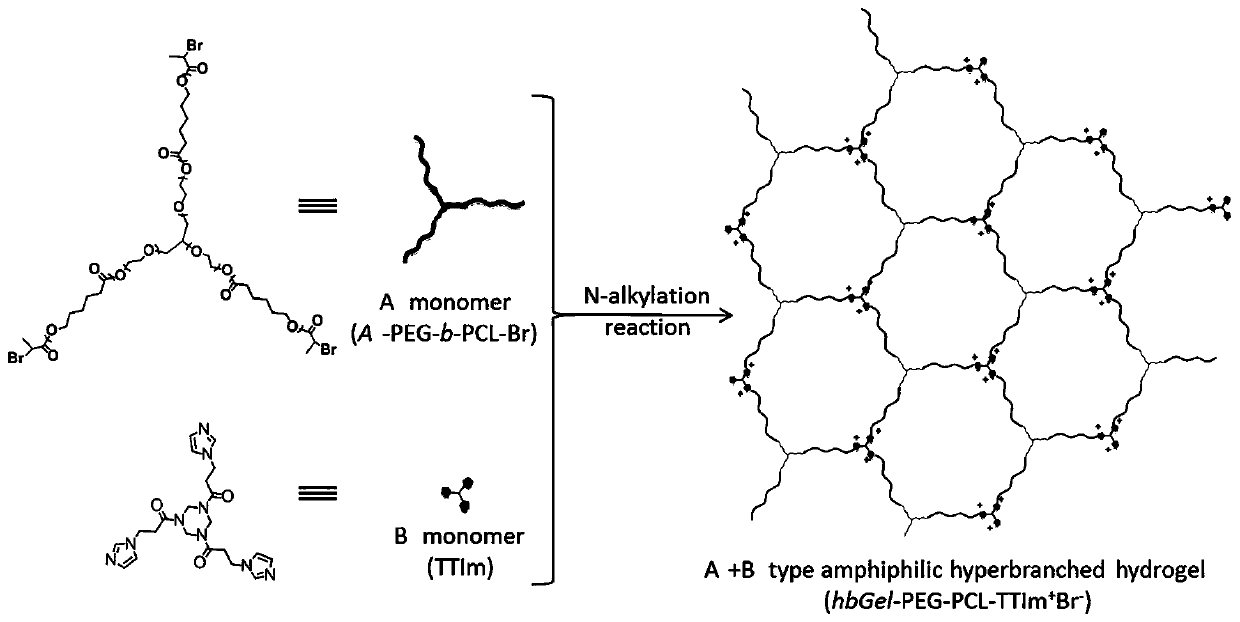
A gel and method of an amphiphilic copolymer network with microscopic pores
An amphiphilic and gel technology, applied in the gel field of amphiphilic copolymer network, can solve problems such as poor adsorption capacity, achieve the effect of improving adsorption performance, promoting interaction, ensuring hydrophobicity and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
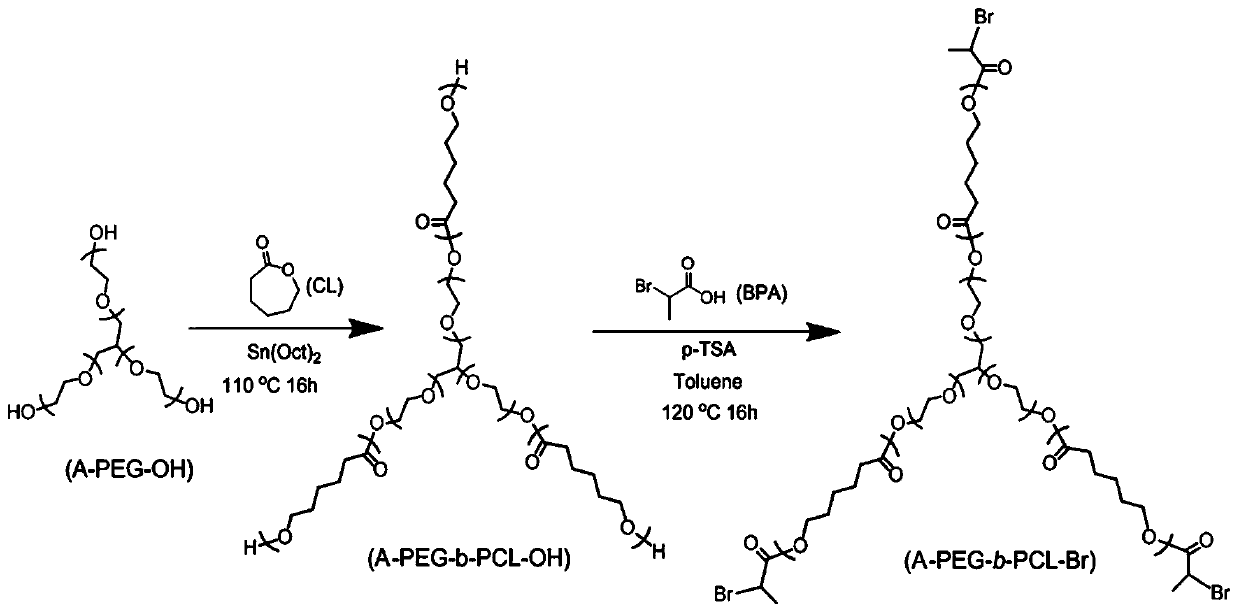
[0058] Experimental Example 1 Synthesis of Polyoxyethyl Glyceryl Ether-b-Polycaprolactone Amphiphilic Block Precursor (A-PEG-b-PCL-OH)
[0059] A-PEG-OH was azeotropically removed with toluene before use. A certain amount of A-PEG-OH and 6-caprolactone CL were added to the reaction flask. After deoxygenation with argon for 40min, Sn(Oct) was added quickly. 2 , after 20 min of deoxygenation with argon, the reaction system was sealed and stirred at 110° C. for 16 h. After the reaction, the reaction system was precipitated in glacial ether, filtered, and the obtained product was vacuum-dried at 40°C to constant weight.
[0060] As shown in Table 1, it is the charging ratio table of each raw material in Experimental Example 1. By adjusting the charging ratio of A-PEG-OH / CL, three kinds of A-PEG-b-PCL-OH precursors with different molecular weights were obtained, and according to The theoretical degree of polymerization of the polymerized units in each arm, respectively named A-PEG...
experiment example 2 3
[0067] Experimental Example 2 Synthesis of Tribromo Amphiphilic A Monomer (A-PEG-b-PCL-Br)
[0068] A certain amount of A-PEG-b-PCL-OH, BPA (α-bromopropionic acid), p-TSA (p-toluenesulfonic acid) and 200 mL of toluene were added to the Dean-Stark device, heated to 120°C, and refluxed. 16h. After the reaction, toluene was evaporated, the crude product was dissolved in dichloromethane, and 2% Na was used in turn. 2 CO 3 3 washes, 2 washes with 5% NaCl solution, and 2 washes with deionized water. Separation with anhydrous MgSO 4 The organic layer was dried, the solvent was removed under reduced pressure, the crude product was precipitated in ice-n-hexane, filtered, and the obtained product was dried under vacuum at 40°C to constant weight.
[0069] The A monomers obtained by the esterification modification corresponding to the precursors 1-3 prepared in Experimental Example 1 are named A-PEG respectively 7 -b-PCL 3 -Br(A monomer 1), A-PEG 7 -b-PCL 5 -Br(A monomer 2) and A...
experiment example 3
[0073] Experimental example 3 Synthesis method of B monomer with imidazole in end group
[0074] It is prepared by Michael addition reaction of 1,3,5-triacryloylhexahydro-1,3,5-triazine (TT) and imidazole (Im), and the specific technical scheme is as follows:
[0075] TT (5 g, 20 mmol) and Im (10 g, 106 mmol) were added to 30 mL of anhydrous methanol and refluxed for 24 h. After the reaction, the reaction system was precipitated in a mixed solvent of ethyl acetate / tetrahydrofuran (1:1 by volume). Then wash twice through dissolution-precipitation (methanol-ethyl acetate / tetrahydrofuran), filter, and vacuum-dry the obtained precipitate to constant weight at room temperature to obtain B monomer (TT Im), as Figure 4 As shown, it is the H nuclear magnetic spectrum of the B monomer with imidazole at the end group prepared in Experimental Example 3 of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| gel fraction | aaaaa | aaaaa |
| gel rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com