24-valence pneumococcus polysaccharide vaccine and identification method thereof
A technology of pneumococcal polysaccharide and pneumococcus, which is applied in the medical field, can solve the problems of difficult formation of precipitation lines, influence of test results, difficulty in implementation of identification tests, etc., achieve good results, and overcome the effect of poor sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The invention provides a pneumococcal polysaccharide, the polysaccharide is identified by immune rate turbidimetry, and the immune rate turbidimetry at least includes contacting the polysaccharide solution with the corresponding antibody to form an antigen-antibody complex, and then the immune rate scatter Nephelometric determination of the reaction rate value of the antigen-antibody complex. In other words, polysaccharides are identified by the rate unit value measured by the specific combination of antigen and antibody to form an antigen-antibody complex.
[0072] The further optimized technical solution of this embodiment is that the 24-valent pneumococcal polysaccharide includes capsular polysaccharides of any 24 serotypes of pneumococcus.
[0073] The further optimized technical solution of this embodiment is that the capsular polysaccharides of any 24 serotypes of pneumococcus include pneumococcal type 1 capsular polysaccharide, pneumococcal type 2 capsular polysa...
Embodiment 2
[0089] The present invention provides a 24-valent pneumococcal polysaccharide vaccine, the pneumococcal polysaccharide vaccine is identified through the turbidimetry of the immune rate, and the turbidimetry of the immune rate at least includes contacting the polysaccharide vaccine with the corresponding antibody to form an antigen-antibody complex , and then the reaction rate value of the antigen-antibody complex was determined by immune rate scattering turbidimetry. In other words, polysaccharide vaccines are identified by using the rate unit value measured by the specific combination of antigen and antibody to form an antigen-antibody complex.
[0090] The further optimized technical solution of this embodiment is that the polysaccharide in the 24-valent pneumococcal polysaccharide vaccine contains capsular polysaccharides of any 24 serotypes of pneumococcus.
[0091] The further optimized technical solution of this embodiment is that the capsular polysaccharides of any 24 s...
Embodiment 3
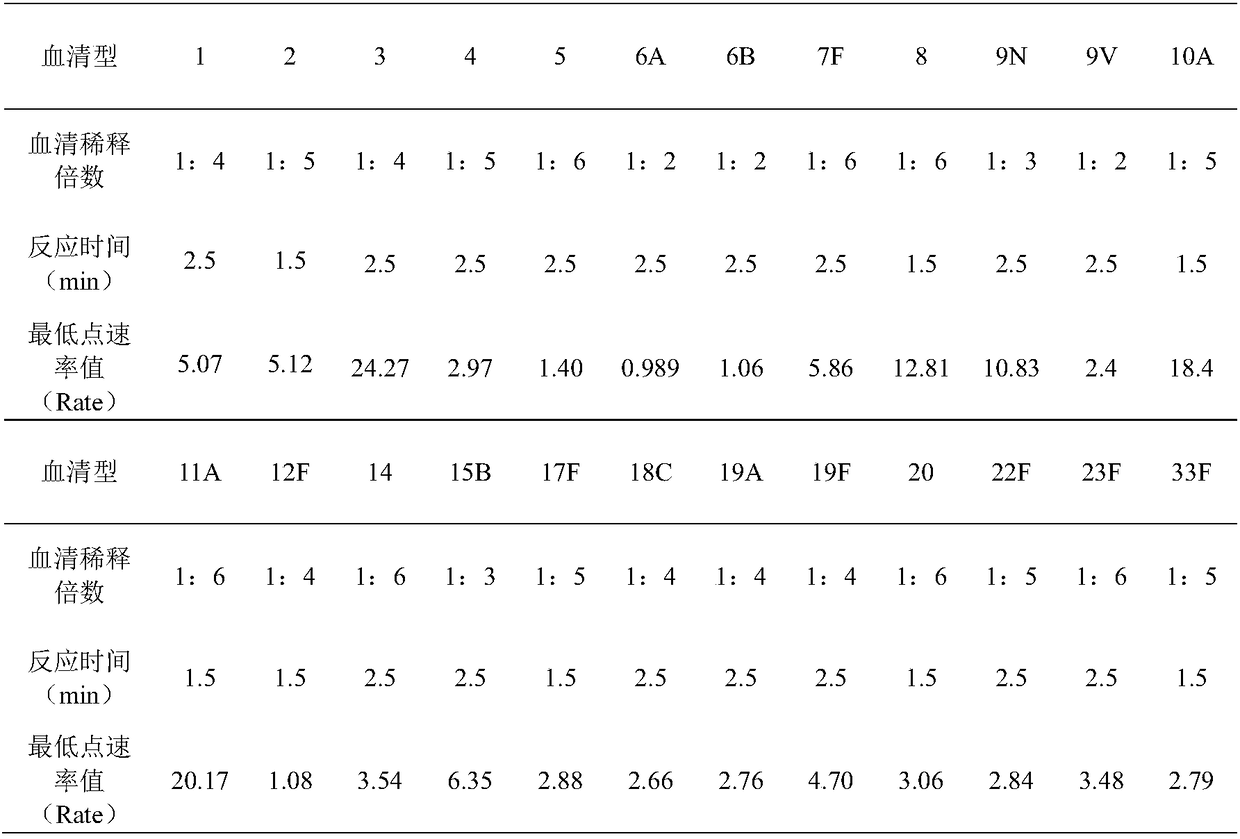
[0108] (1) Sample source: pneumococcal serotypes 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F capsular polysaccharide standard products were purchased from ATCC, pneumococcal serotypes 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 9N, 10A, 11A, 12F, 14 , 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F antisera were purchased from the Danish National Serum Institute.
[0109] (2) Prepare the following pneumococcal polysaccharide stock solution and polysaccharide vaccine according to the method published by another invention patent of our company CN201010129404.2: pneumococcal type 1 capsular polysaccharide, pneumococcal type 2 capsular polysaccharide, pneumococcal type 3 capsular polysaccharide, pneumonia Pneumococcus type 4 capsular polysaccharide, pneumococcal type 5 capsular polysaccharide, pneumococcal type 6A capsular polysaccharide, pneumococcal type 6B capsular polysaccharide, pneumococcal type 7F capsular polysaccharide, pneumococca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com