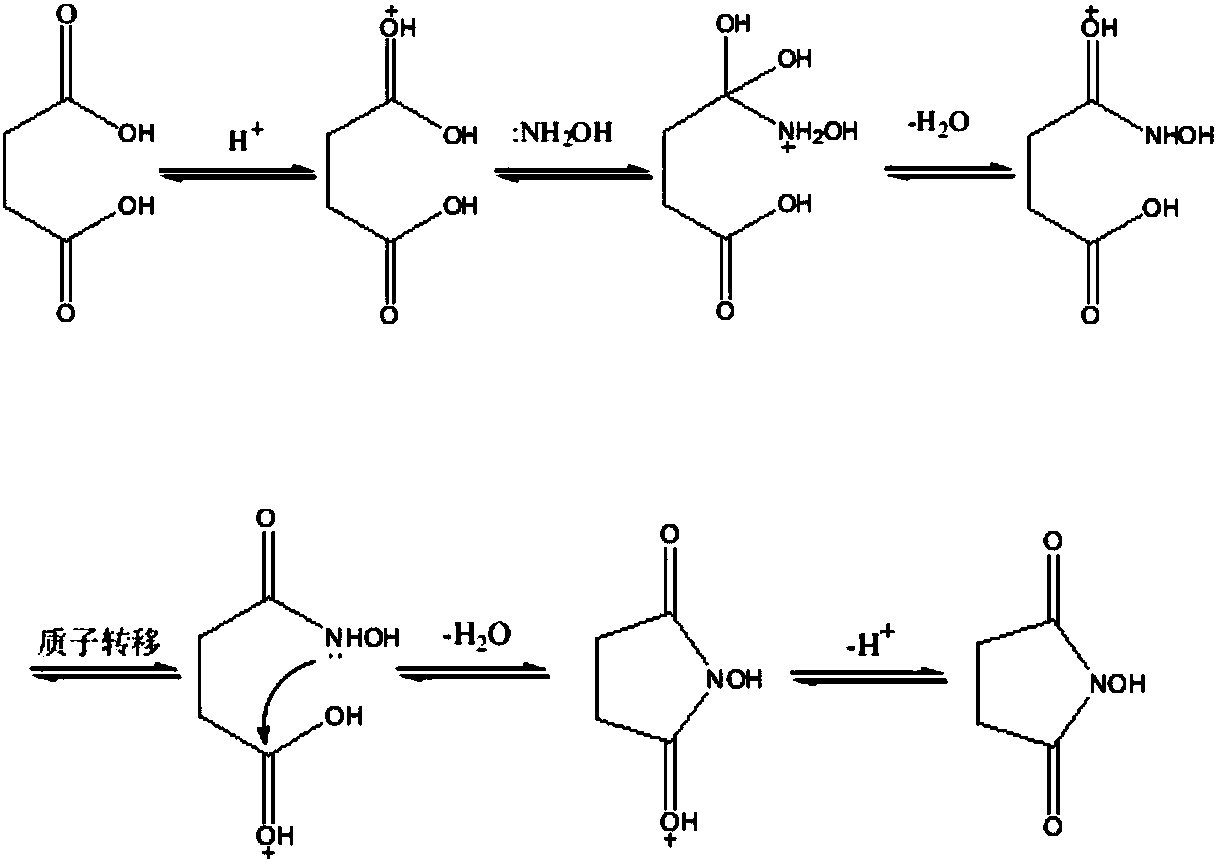
Preparation method of N-hydroxysuccinimide
A technology of hydroxysuccinimide and hydroxylamine, which is applied in organic chemistry and other fields, and can solve problems such as high safety hazards, high chemical dangers, and high prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] First, 160 grams of succinic acid and 59.5 grams of sodium hydroxide are reacted to generate succinic acid sodium salt.
[0035] Install a mechanical stirrer, a spherical condenser with a water separator, and a thermometer in a four-neck round bottom flask, and then add 400 mL of toluene. In an oil bath (temperature 140° C.), heat and reflux for dehydration until no water comes out. Cool the reaction system to room temperature, continue to add succinic acid sodium salt, 144 g of hydroxylamine sulfate, and 4.5 g of boric acid, and continue to heat and reflux for dehydration until the reaction is basically no dehydration. Cool the reaction system, transfer the toluene, add 400ml of absolute ethanol to reflux to extract the product for 20-30 minutes, cool the reaction system and remove the by-product sodium sulfate by suction filtration, and wash the filter cake three times with absolute ethanol.
[0036] Concentrate the mother liquor obtained above under normal pressure,...
Embodiment 2
[0038]Install a mechanical stirrer, a spherical condenser with a water separator, and a thermometer in a four-neck round bottom flask, and then add 400 mL of toluene. In an oil bath (temperature 140° C.), heat and reflux for dehydration until no water comes out. Cool the reaction system to room temperature, continue to add 160 grams of succinic acid, 144 g of hydroxylamine sulfate, 4.5 g of boric acid, and 59.5 grams of sodium hydroxide, and continue to heat and reflux for dehydration until the reaction is basically no dehydration. Cool the reaction system, transfer the toluene, add 400ml of absolute ethanol to reflux to extract the product for 20-30 minutes, cool the reaction system and remove the by-product sodium sulfate by suction filtration, and wash the filter cake three times with absolute ethanol.
[0039] Concentrate the mother liquor obtained above under normal pressure, and change the distillation under reduced pressure to remove ethanol as much as possible when the...
Embodiment 3
[0041] Succinic acid 1180g, sodium hydroxide 440g, hydroxylamine sulfate 1066g, boric acid 33g, succinic acid was added twice to produce product NHS830g, yield 72%, liquid chromatography detection content 99.48%, melting point 95-97 ℃.
[0042] Specific operation: 649g of succinic acid, 440g of sodium hydroxide, 2000mL of toluene, reflux dehydration for 1 hour, then add 531g of succinic acid, 1066g of hydroxylamine sulfate, 33g of boric acid, and 1000ml of toluene for dehydration, and the dehydration time is 6.5 hours. Pour out the toluene, then dissolve the solid with 2000ml of absolute ethanol (heat to reflux for 30min), condense and filter with suction, and wash the filter cake with absolute ethanol three times. Evaporate the ethanol to dryness under reduced pressure, add 2000ml of ethyl acetate to heat and extract the product, reflux for 30min, stand still and cool down to 60°C, separate the ethyl acetate and continue to cool down to room temperature, return the mother liqu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com