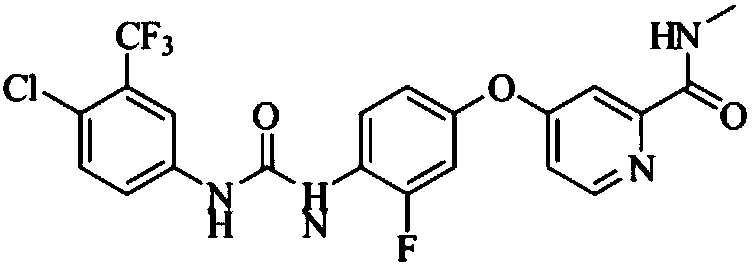
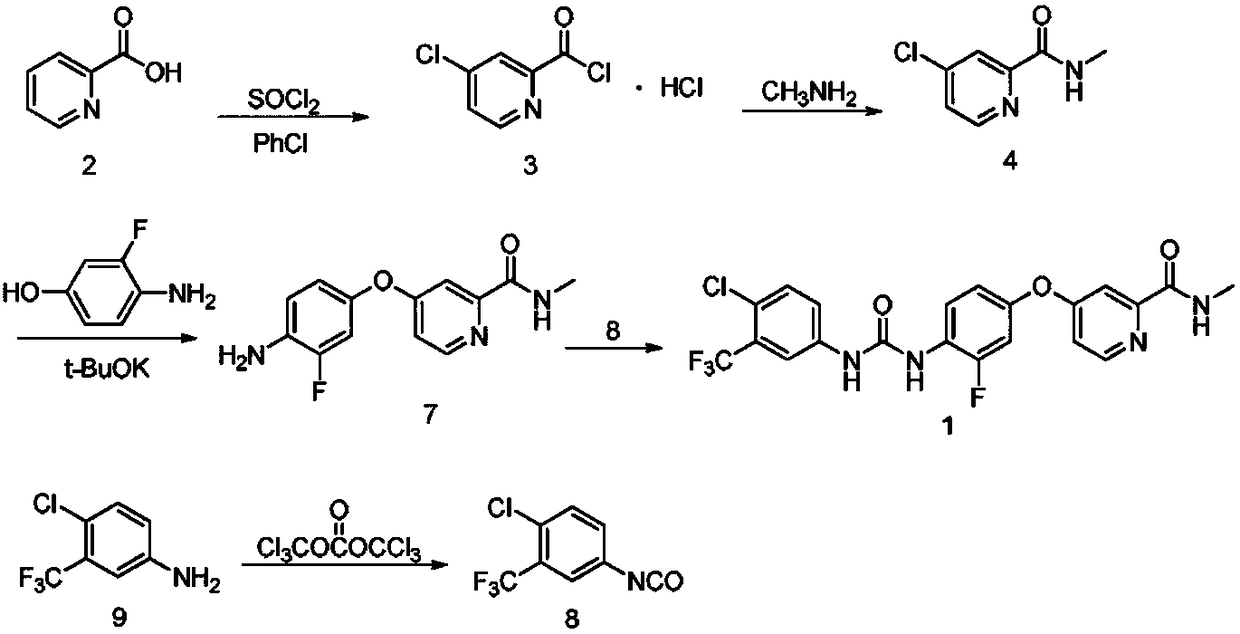
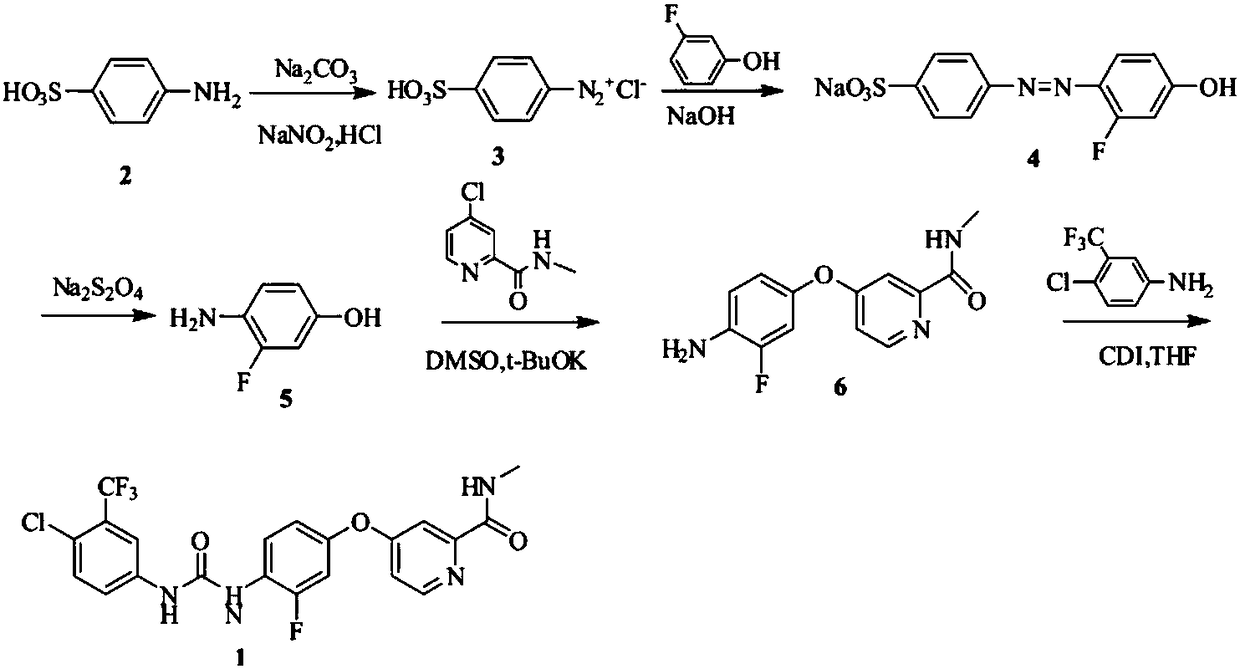
Preparation method of regorafenib
A technology of regorafenib and quantity ratio, applied in the field of preparation of regorafenib, can solve the problems of unsuitable industrial production, expensive catalyst, difficult operation, etc., and achieves reduction of production cost, saving of solvent consumption cost, and method conversion rate. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0024] The synthesis of embodiment 1-1 intermediate I
[0025] In a 100mL three-neck flask equipped with a magnetic stirring device, a thermometer and a reflux condenser, add 0.09mol of 3-fluoro-4-nitrophenol, heat and melt, add 0.10mol of KOH, and react for 10min under stirring. Add 0.10mol of 4-chloro-N-methylpyridine-2-carboxamide to the reaction system, and react with temperature control; the end point is detected by TLC, and the reaction ends in 1.5h; hot 5% NaOH solution is added to the reaction system, and the temperature is kept at 80°C After washing 3 times at ~85°C, and washing 3 times with water at the same temperature, the reaction mixture was poured into cold water while it was hot, cooled, filtered, and dried to obtain a solid. Mix the obtained solid, 1 g of activated carbon for sugar, 0.12 mmol of ferric chloride, and 50 mL of methanol in a 250 mL four-necked flask, and add 0.33 mol of 85% hydrazine hydrate dropwise at reflux temperature for 1 hour. After the ad...
Embodiment 1-2
[0026] The synthesis of embodiment 1-2 intermediate I
[0027]In a 100mL three-neck flask equipped with a magnetic stirring device, a thermometer and a reflux condenser, add 0.09mol of 3-fluoro-4-nitrophenol, heat and melt, add 0.10mol of KOH, and react for 10min under stirring. Add 0.10mol of 4-chloro-N-methylpyridine-2-carboxamide to the reaction system, and react with temperature control; the end point is detected by TLC, and the reaction ends in 1.5h; hot 5% NaOH solution is added to the reaction system, and the temperature is kept at 80°C After washing at ~85°C for 3 times and with water at the same temperature for 3 times, the reaction mixture was poured into cold water while it was hot, cooled, filtered, and dried to obtain a solid. Mix the obtained solid, 1g of activated carbon for sugar, 0.12mmol of ferric chloride, and 50mL of methanol in a 250mL four-necked flask, and add 0.30mol of 85% hydrazine hydrate dropwise at reflux temperature for 1 hour. After the dropwise ...
Embodiment 1-3
[0028] Synthesis of Example 1-3 Intermediate I
[0029] In a 100mL three-necked flask equipped with a magnetic stirring device, a thermometer and a reflux condenser, add 0.08mol of 3-fluoro-4-nitrophenol, heat and melt, add 0.10mol of KOH, and react for 10min under stirring. Add 0.09mol of 4-chloro-N-methylpyridine-2-carboxamide to the reaction system, and react with temperature control; the end point is detected by TLC, and the reaction ends in 1.5h; hot 5% NaOH solution is added to the reaction system, and the temperature is kept at 80°C After washing at ~85°C for 3 times and with water at the same temperature for 3 times, the reaction mixture was poured into cold water while it was hot, cooled, filtered, and dried to obtain a solid. Mix the obtained solid, 1g of activated carbon for sugar, 0.12mmol of ferric chloride, and 50mL of methanol in a 250mL four-necked flask, and add 0.30mol of 85% hydrazine hydrate dropwise at reflux temperature for 1 hour. After the dropwise addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com