Sodium doped "bow tie" nickel-cobalt pyrophosphate composite electrode material and preparation method thereof
A composite electrode, bow-tie technology, used in hybrid capacitor electrodes, hybrid/electric double-layer capacitor fabrication, nanotechnology for materials and surface science, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] ①Add 0.0550g nickel nitrate hexahydrate, 0.0550g cobalt nitrate hexahydrate, 0.776g sodium tartrate and 0.150g triammonium phosphate into 20.0mL distilled water, stir and mix well, transfer the above reaction solution to the reaction kettle, and heat Under the condition of hydrothermal reaction for 24 hours, after the completion of the reaction, naturally cool to room temperature, the product is centrifuged, washed several times with absolute ethanol and distilled water and dried, and the "bow tie" precursor compound can be obtained.
[0022] ②Put the "bow tie" precursor compound prepared in step ① on a clean and dry porcelain boat, put it into a tube furnace, and calcinate it at 650°C for 0.5h in an air atmosphere. After the reaction, the tube furnace The furnace is naturally cooled to room temperature, and the sodium-doped "bowtie"-shaped nickel-cobalt pyrophosphate composite electrode material can be finally obtained.
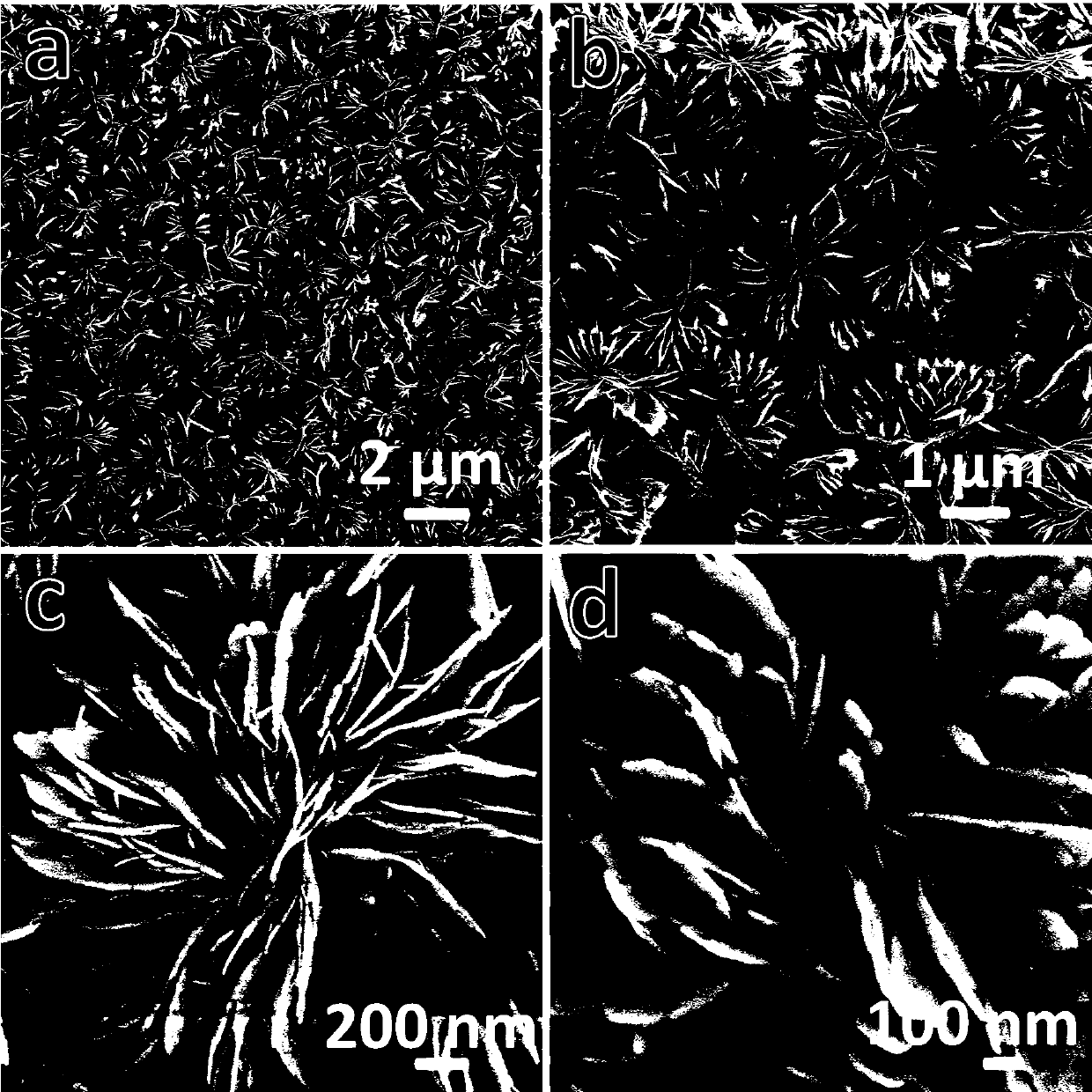
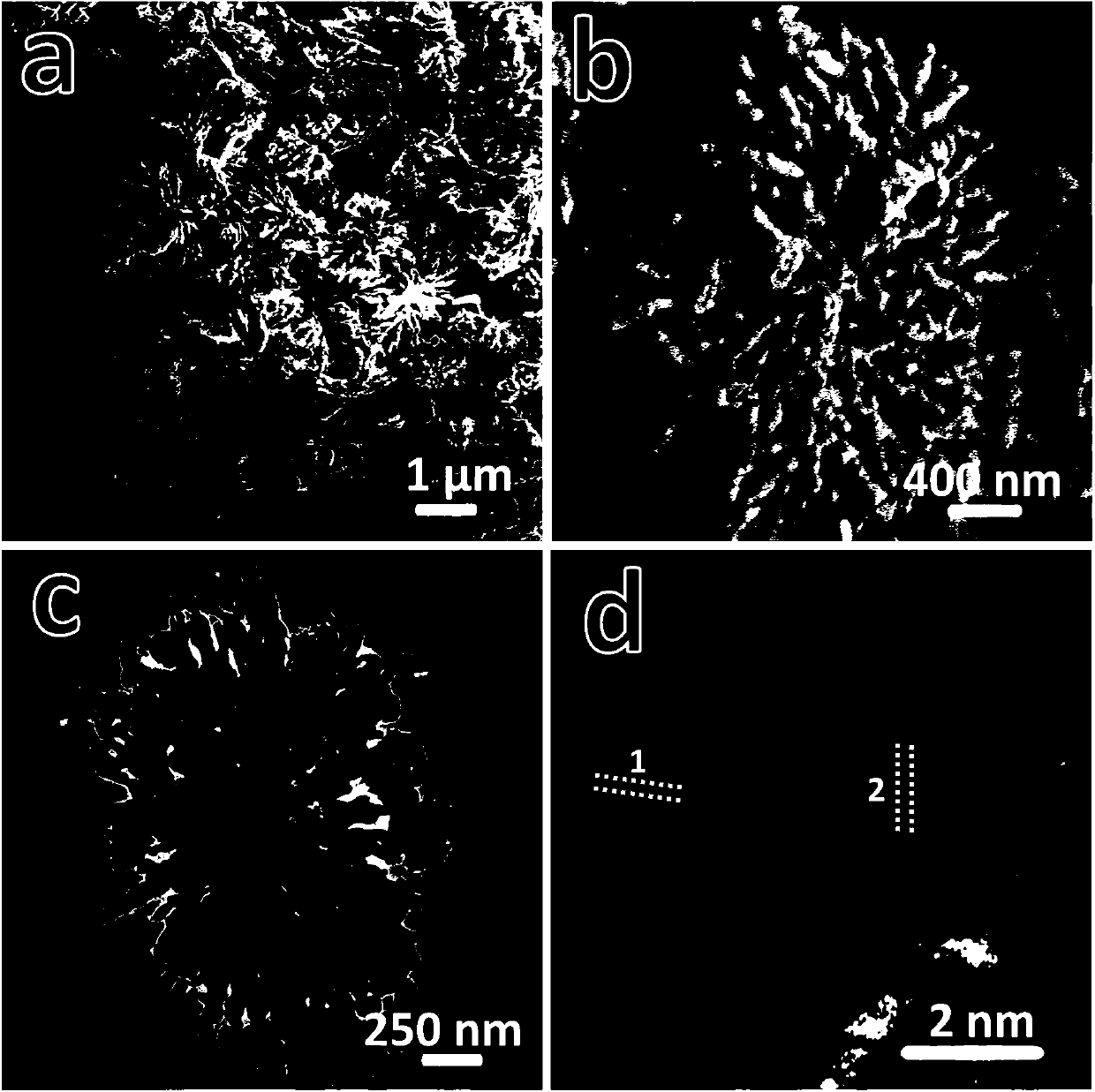
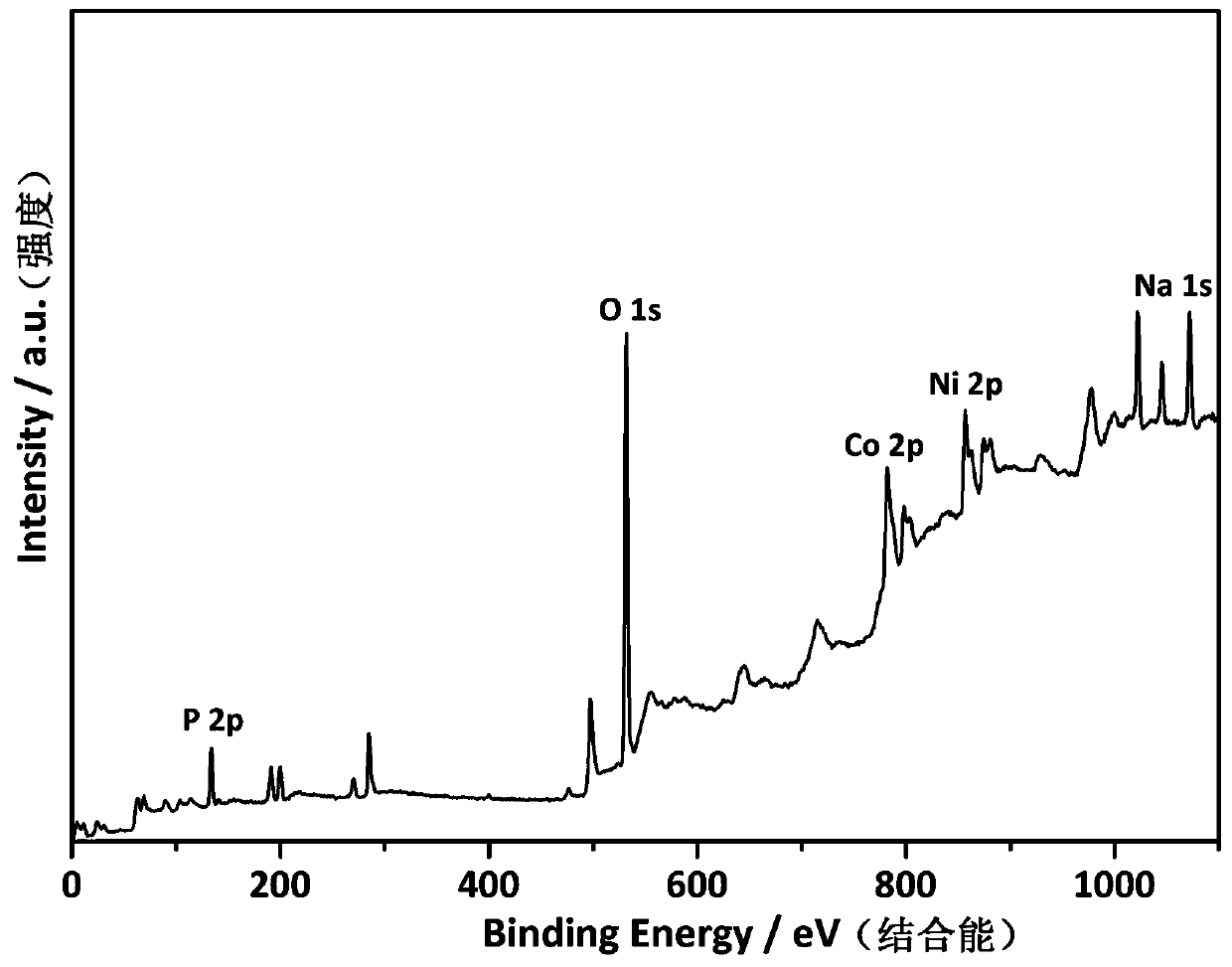
[0023] attached figure 1 The field emission sc...
Embodiment 2
[0026] ① Add 0.0291g nickel nitrate hexahydrate, 0.0869g cobalt nitrate hexahydrate, 0.970g sodium tartrate and 0.150g triammonium phosphate into 20.0mL distilled water, stir and mix well, transfer the above reaction solution to the reaction kettle, and heat Under the condition of hydrothermal reaction for 24 hours, after the reaction, naturally cool to room temperature, the product is centrifuged, washed several times with absolute ethanol and distilled water and dried, and the "bow tie" precursor compound can be obtained.
[0027] ②Put the "bow tie" precursor compound prepared in step ① on a clean and dry porcelain boat, put it into a tube furnace, and calcinate it at 600°C for 0.5h in an air atmosphere. After the reaction, tube The furnace is naturally cooled to room temperature, and the sodium-doped "bowtie"-shaped nickel-cobalt pyrophosphate composite electrode material can be finally obtained.
Embodiment 3
[0029] ① Add 0.0872g nickel nitrate hexahydrate, 0.0288g cobalt nitrate hexahydrate, 1.164g sodium tartrate and 0.300g triammonium phosphate into 20.0mL distilled water, stir and mix well, transfer the above reaction solution to the reaction kettle, and heat Under the condition of hydrothermal reaction for 24 hours, after the reaction, naturally cool to room temperature, the product is centrifuged, washed several times with absolute ethanol and distilled water and dried, and the "bow tie" precursor compound can be obtained.
[0030] ②Put the "bow tie" precursor compound prepared in step ① on a clean and dry porcelain boat, put it into a tube furnace, and calcinate it at 550°C for 0.5h in an air atmosphere. After the reaction, the tube furnace The furnace is naturally cooled to room temperature, and the sodium-doped "bowtie"-shaped nickel-cobalt pyrophosphate composite electrode material can be finally obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com