Preparation method for benzbromarone
A technology of benzbromarone and bromine salt, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as equipment corrosion, high production cost, and high price, and achieve the effects of avoiding the formation of impurities, improving the utilization rate of bromine, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Weigh 2mmol of 15-crown-5, 4.4mmol of LiBr and 4.4mmol of NaClO, dissolve them in 7mL of water respectively, mix and stir the three solutions at room temperature for 30min, filter, wash with water 3 times, and dry to obtain yellow Solid 15-crown-5·bromine, yield 87%.
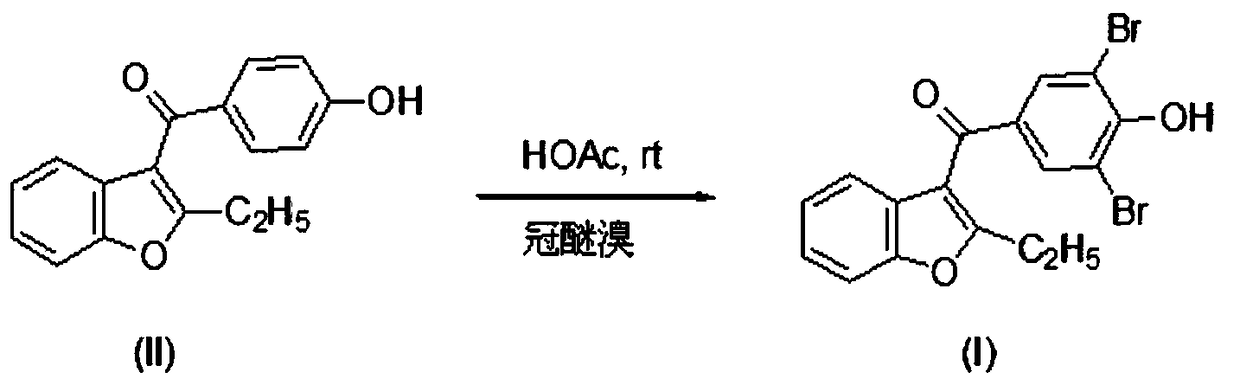
[0016] Dissolve 2.0 mmol of 15-crown-5 bromine in 15 mL of HOAc, slowly add 1 mmol of (4-hydroxyphenyl)-(2-ethylbenzofuran-3-yl)methanone under stirring 10 mL of HOAc solution. React at room temperature for 10 hours, raise the temperature to 40°C, stir for 30 minutes, cool to room temperature, filter, and recrystallize from 95% ethanol to obtain benzbromarone with a yield of 91%. mp151-152°C.
[0017] During the reaction process, 10% LiOH solution was used to absorb the HBr produced by the reaction to neutrality to prepare LiBr, and the content of LiBr was determined by silver nitrate titration.
[0018] The filtrate was evaporated to remove HOAc under reduced pressure, and 15 mL of water was added to ...
Embodiment 2
[0020] Weigh 2mmol of dibenzo-18-crown-6 and 4.4mmol of NaBr respectively, dissolve them in 7mL of water, and then weigh 4.4mmol of 30% H 2 o 2 , at room temperature, the three solutions were mixed and stirred for 30 min, filtered, washed 3 times with water, and dried to obtain a yellow solid dibenzo-18-crown-6 bromide with a yield of 88%.
[0021] Dissolve 2.03 mmol of dibenzo-18-crown-6 bromide in 15 mL of HOAc, slowly add 1 mmol of (4-hydroxyphenyl)-(2-ethylbenzofuran-3-yl) under stirring ) ketone in 10 mL of HOAc solution. React at room temperature for 10 hours, raise the temperature to 40°C, stir for 30 minutes, cool to room temperature, filter, and recrystallize from 95% ethanol to obtain benzbromarone with a yield of 94%. mp152-153°C.
[0022] During the reaction process, the HBr produced by the reaction was absorbed with 20% NaOH solution to neutrality to prepare NaBr, and the content of NaBr was determined by silver nitrate titration.
[0023] The filtrate was eva...
Embodiment 3
[0025] Weigh 2mmol of dicyclohexane and 18-crown ether-6, 2.2mmol of MgBr 2 and 4.4mmol of t-BuOOH were dissolved in 10mL of water respectively. At room temperature, the three solutions were mixed and stirred for 30min, filtered, washed 3 times with water, and dried to obtain a yellow solid dicyclohexane and 18-crown-6 bromide , yield 90%.
[0026] Dissolve 2.0 mmol of dicyclohexane 18-crown-6 bromide in 15 mL of HOAc, slowly add 1 mmol of (4-hydroxyphenyl)-(2-ethylbenzofuran-3- base) ketone in 10 mL of HOAc solution. React at room temperature for 10 hours, raise the temperature to 40°C, stir for 30 minutes, cool to room temperature, filter, and recrystallize from 95% ethanol to obtain benzbromarone with a yield of 91.5%. mp152-153°C.
[0027] During the reaction, use 15% Mg(OH) 2 The solution absorbs the HBr produced by the reaction to neutrality and prepares MgBr 2 , Determination of MgBr by silver nitrate titration 2 content.
[0028] The filtrate was evaporated unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com