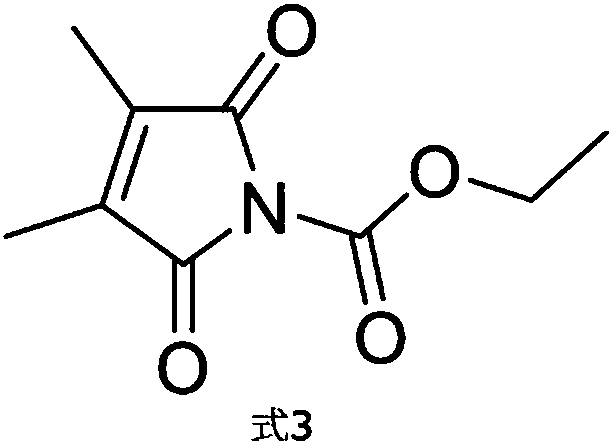
Synthesis method of high-purity N-ethoxycarbonyl-2, 3-disubstituted maleimide
A technology of dimethyl butenedimide and butenedimide is applied in the field of N-ethoxycarbonyl-2 and can solve the problem of N-ethoxycarbonyl-2,3-disubstituted butenedioyl The purity of imine is not high enough, and it cannot meet the development requirements of enzyme-specific kits, so as to achieve the effect of simple operation, good market application value and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthetic method of a kind of high-purity N-ethoxycarbonyl-2,3-disubstituted butenedimide of the present embodiment comprises the following steps:
[0023] (1) Preparation of 2,3-dimethylbutenediimide
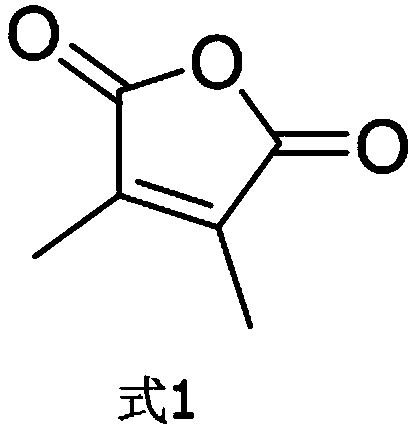
[0024] A. Dissolve 1.0g of 3,4-dimethylfuran-2,5-dione (structural formula shown in formula 1) in 10mL of DMF, add 17mL of hexamethyldisilazane HMDS and 1.7mL of Methanol was added and reacted at room temperature for 1 h, and then the corresponding solvent was evaporated to dryness using a rotary evaporator;
[0025]
[0026] B. Add 4mL DMF, 17mL HMDS and 1.7mL methanol to react for 7 hours, then add 65mL water and 45mL ethyl acetate for extraction, and extract twice;
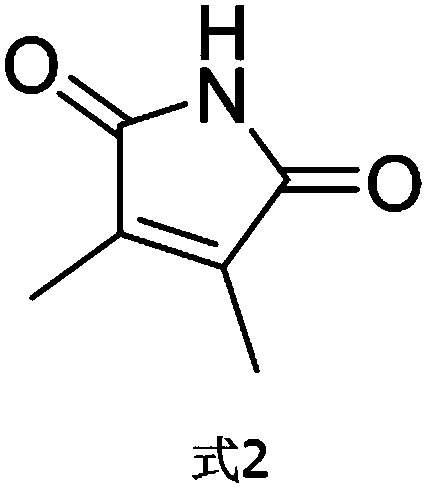
[0027] C. Merge the organic phases, and wash the extract with water and saturated brine, then dry it with anhydrous sodium sulfate drying method, evaporate the solvent, and dry it with an oil pump to obtain 0.9 g of white solid, namely 2,3-dimethyl Butene imide (structural formula shown in formul...
Embodiment 2
[0034] The synthetic method of a kind of high-purity N-ethoxycarbonyl-2,3-disubstituted butenedimide of the present embodiment comprises the following steps:
[0035] (1) Preparation of 2,3-dimethylbutenediimide
[0036] A. Dissolve 1.2g of 3,4-dimethylfuran-2,5-dione (structural formula shown in Formula 1) in 12mL of DMF, add 19mL of hexamethyldisilazane HMDS and 1.9mL of Methanol was added and reacted at room temperature for 3 hours, and then the corresponding solvent was evaporated to dryness using a rotary evaporator;
[0037]
[0038] B. Add 6 mL of DMF, 19 mL of HMDS and 1.9 mL of methanol to react for 9 hours, then add 75 mL of water and 55 mL of ethyl acetate for extraction, and extract 4 times;
[0039] C. Merge the organic phases, and wash the extract with water and saturated brine, then dry it with anhydrous sodium sulfate drying method, evaporate the solvent, and dry it with an oil pump to obtain 1.1 g of white solid, namely 2,3-dimethyl Butene imide (structur...
Embodiment 3
[0046] The synthetic method of a kind of high-purity N-ethoxycarbonyl-2,3-disubstituted butenedimide of the present embodiment comprises the following steps:
[0047] (1) Preparation of 2,3-dimethylbutenediimide
[0048] A. Dissolve 1.1g of 3,4-dimethylfuran-2,5-dione (structural formula shown in formula 1) in 11mL of DMF, add 18mL of hexamethyldisilazane HMDS and 1.8mL of Methanol was added and reacted at room temperature for 2 hours, and then the corresponding solvent was evaporated to dryness using a rotary evaporator;
[0049]
[0050] B. Add 5mL DMF, 18mL HMDS and 1.8mL methanol to react for 8 hours, then add 70mL water and 50mL ethyl acetate for extraction, and extract 3 times;
[0051] C. Merge the organic phases, and wash the extract with water and saturated brine, then dry it with anhydrous sodium sulfate drying method, evaporate the solvent, and dry it with an oil pump to obtain 1.0 g of white solid, namely 2,3-dimethyl Butylene imide (structural formula shown in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com