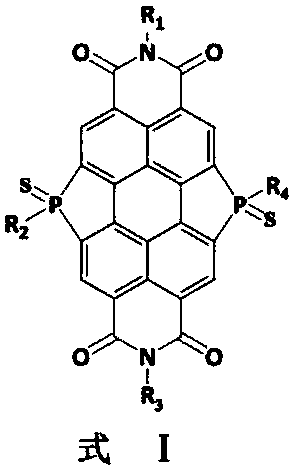
Bay-position organic phosphine bridged perylene bisimide containing phosphorus-sulfur bond structure and preparation method thereof
A technology of biperyleneimide and tetrabromoperyleneimide, which is applied in the field of organic chemical synthesis, can solve problems affecting device performance, molecular synthesis and application research limitations, poor solubility of peryleneimide and its derivatives, etc. Achieve the effects of simple preparation reaction conditions, increased solubility and photothermal stability, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
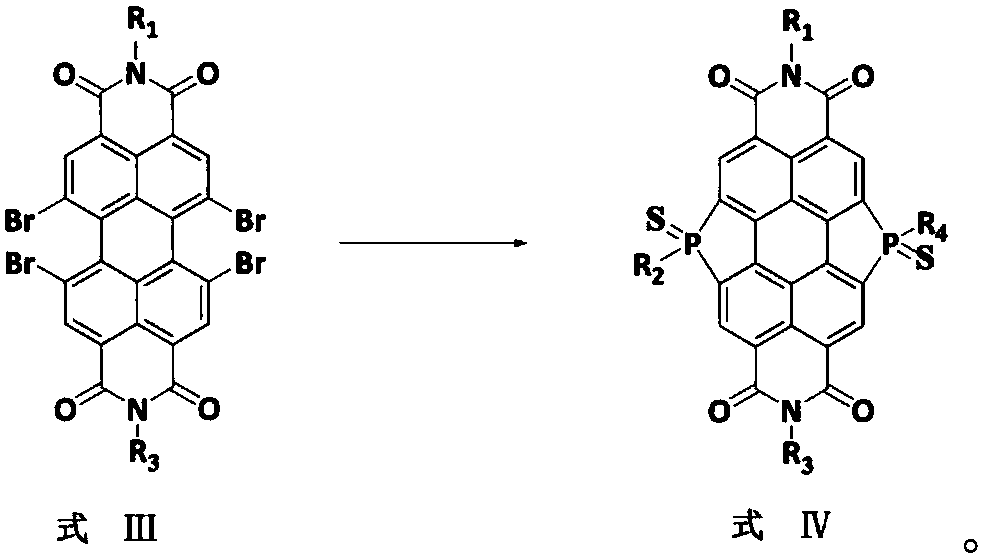
[0024] Another aspect of the present invention provides a method for preparing bay-position organic phosphine bridged perylene imide with a phosphorus-sulfur bond structure. In an exemplary embodiment of the preparation method of the bay-position organic phosphine bridged perylene imide containing phosphorus-sulfur bond structure of the present invention, the preparation method may include:
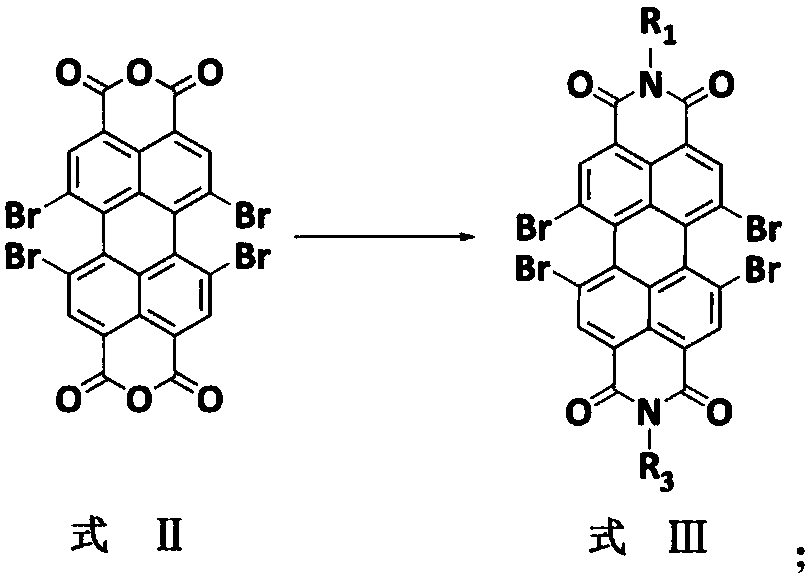
[0025] Step S01, adding tetrabromoperylenetetracarboxylic dianhydride with the structure of formula (II) into the solvent, adding primary amine compounds under stirring conditions, heating the reaction under an inert protective atmosphere, and then cooling to room temperature, removing the solvent The obtained crude product is purified to obtain a tetrabromoperylene imide compound having the structure of formula (III). The reaction formula can be:
[0026]
[0027] In this example, the primary amine compound may include alkylamines, alkylamines containing substituents, cycloalkyls, cycloalkyls...
example 1
[0057] (1) Add 1,6,7,12-tetrabromo-3,4,9,10-perylenetetracarboxylic dianhydride (1.4g, 2mmol) with the structure of formula (II) to 30mL refined anhydrous In the propionic acid, add n-dodecylamine (1.1 g, 6 mmol) with stirring. Under the protection of nitrogen, the reaction was heated to 120°C for 12 hours, and then the reaction was cooled to room temperature. The propionic acid was removed by a rotary evaporator. The crude product obtained was purified by a silica gel column (300-400 mesh) with a solution containing toluene as the eluent , To obtain 1.6 g of 1,6,7,12-tetrabromo-3,4,9,10-perylenetetracarboxylic diimide compound with a chemical structure represented by formula (III-1), with a yield of 76.7%. Mass spectrometric characterization data of the compound of formula (Ⅲ-1): ESI-MS(M+H)(m / z):1043.1,calcd forC 48 H 55 Br 4 N 2 O 4 (m / z),1042.1,Anal.Calcd for C 48 H 54 Br 4 N 2 O 4 .
[0058] (2) Add the perylene imide (1.04g, 1.0mmol) of the chemical structure represented b...
example 2
[0061] (1) Add 1,6,7,12-tetrabromo-3,4,9,10-perylenetetracarboxylic dianhydride (1.4g, 2mmol) with the structure of formula (II) to 30mL refined anhydrous In the propionic acid, add n-dodecylamine (1.1 g, 6 mmol) with stirring. Under the protection of nitrogen, the reaction was heated to 120°C for 12 hours, and then the reaction was cooled to room temperature. The propionic acid was removed by a rotary evaporator. The crude product obtained was purified by a silica gel column (300-400 mesh) with a solution containing toluene as the eluent , To obtain 1.6 g of 1,6,7,12-tetrabromo-3,4,9,10-perylenetetracarboxylic diimide compound with a chemical structure represented by formula (III-1), with a yield of 76.7%. Mass spectrometric characterization data of the compound of formula (Ⅲ-1): ESI-MS(M+H)(m / z):1043.1,calcd for C 48 H 55 Br 4 N 2 O 4 (m / z),1042.1,Anal.Calcd for C 48 H 54 Br 4 N 2 O 4 .
[0062] (2) Take the peryleneimide (1.04g, 1.0mmol) of the chemical structure represented ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com