Method for preparing calcium carbonate of different crystal forms
A technology of calcium carbonate and crystal form, applied in the field of calcium carbonate, can solve problems such as high energy consumption, difficult condition control, and difficult recycling of additives, and achieve the effect of industrialization and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Raw materials include: CO 2 (Xiamen Tongan Air Separation Special Gas Plant, purity>98%), calcium chloride (Sinopharm Chemical Reagent Co., Ltd., purity 96%), 1,8-diazacyclo[5,4,0]undecene -7 (DBU, Shanghai Dibo Biotechnology Co., Ltd., purity 99%).
[0025] DBU and water are mixed in a volume ratio of 1:1, stirred and bubbled into carbon dioxide at room temperature for about 60 minutes, and then the solution is mixed with a certain amount of calcium chloride aqueous solution (the total concentration of calcium chloride after mixing is 0.09mol / L) React at 30.0°C for about 10 minutes, centrifuge and wash the solid product generated by the reaction, and dry it in vacuum for characterization. Sodium hydroxide solution was added to the filtrate, the layers were allowed to stand, and the upper oil phase was reused.
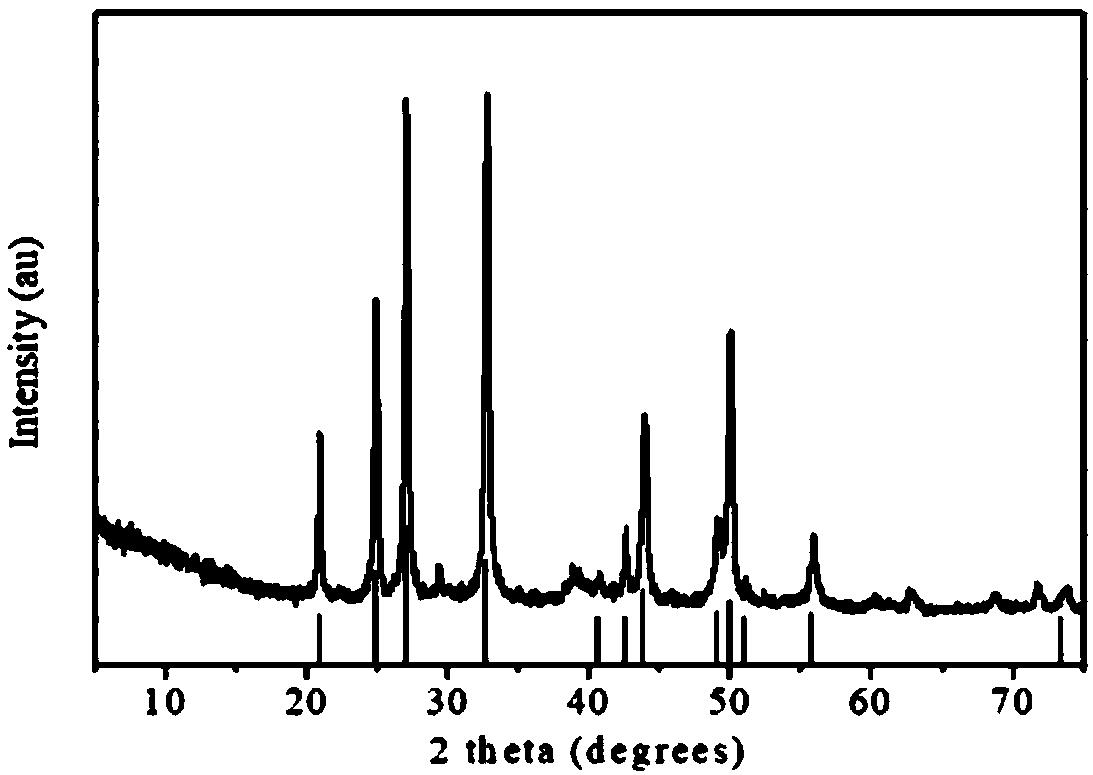
[0026] figure 1 The SEM image of the given product; figure 2 The XRD figure of this product is given, and analysis with this figure can know that the fina...
Embodiment 2
[0028] The raw materials and method are the same as in Example 1, except that the total concentration of the calcium chloride aqueous solution is 1.6 mol / L and reacted at 70.0° C. for 10 min. image 3 The electron microscope picture of the product given; Figure 4 An XRD pattern of the product is given, and analysis of the pattern shows that the final product contains 95.0% aragonitic calcium carbonate, and the remainder is calcite calcium carbonate (5.0%).
Embodiment 3
[0030] The raw material different from Example 1 was N,N-dimethylcyclohexylamine (98%, Aladdin Industries).
[0031] N,N-Dimethylcyclohexylamine and water are mixed in a volume ratio of 1:1 with stirring and bubbling carbon dioxide at room temperature for about 60 minutes, and then the solution is mixed with a certain amount of calcium chloride aqueous solution (chlorination The total concentration of calcium after mixing is 1.0mol / L) and react at 40.0°C for about 120min. The solid product generated by the reaction is centrifuged and washed, dried in vacuum, and characterized. Sodium hydroxide solution was added to the filtrate, the layers were allowed to stand, and the upper oil phase was reused.
[0032] Figure 5 The electron microscope picture of the product given; Figure 6 The XRD figure of this product is given, and analysis with this figure can know that the final product contains 98.9% of vaterite calcium carbonate, and the rest is calcite calcium carbonate (1.1%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com