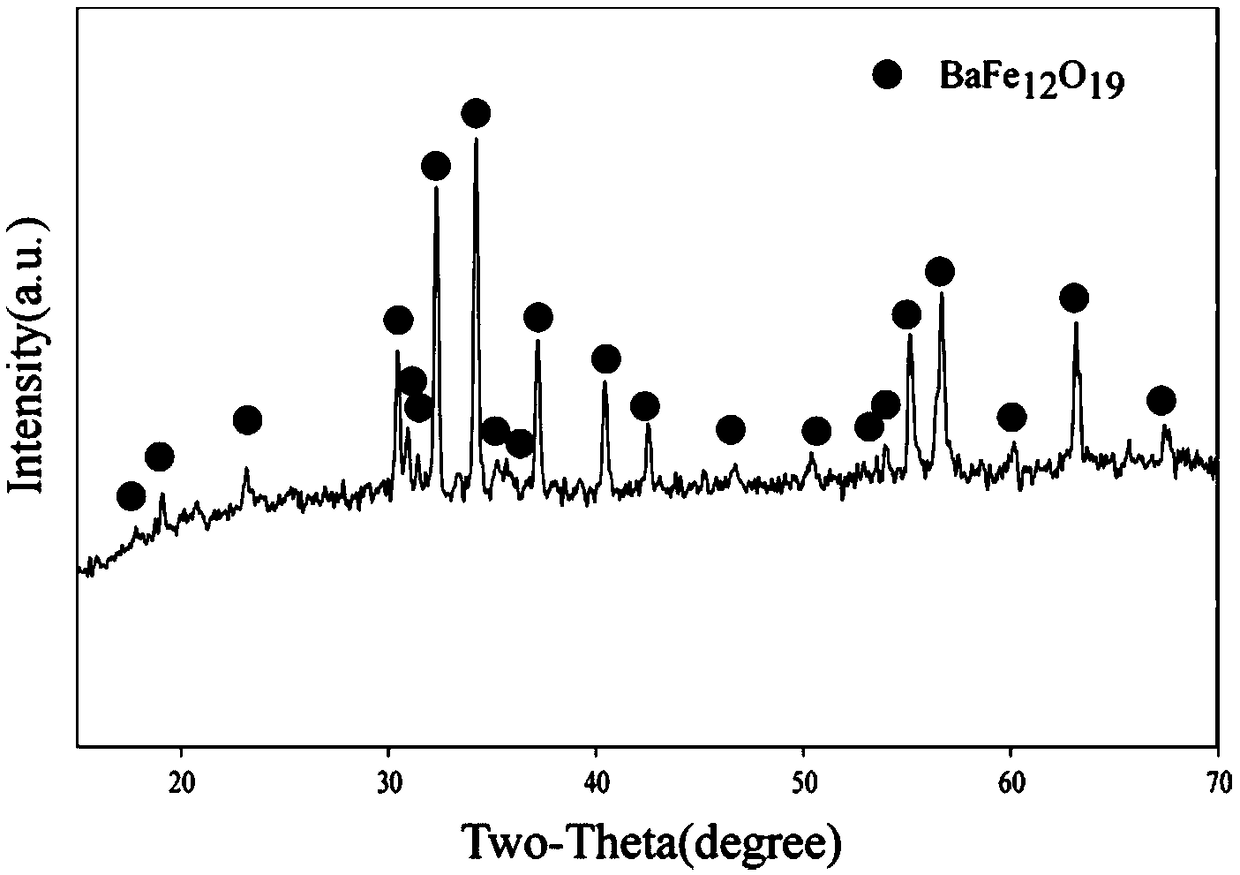
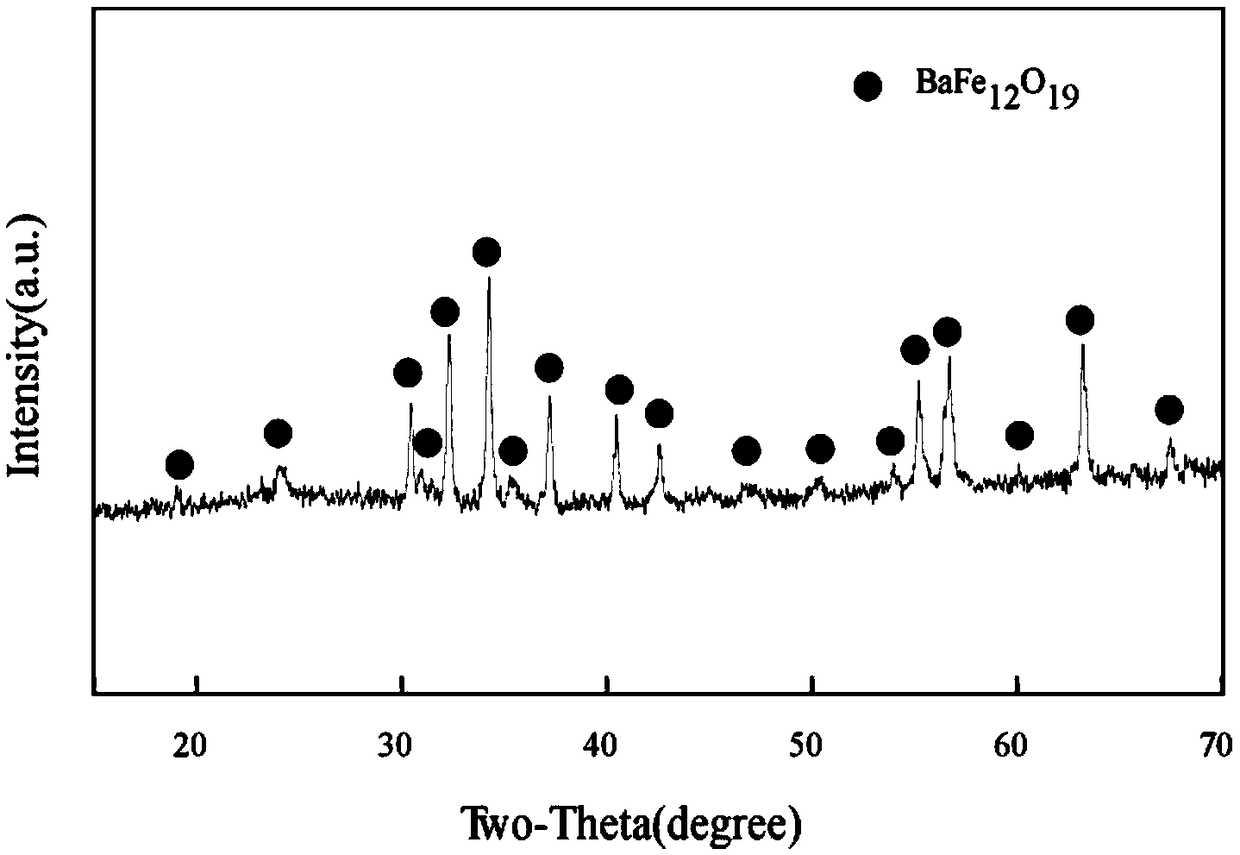
Preparation method of high-purity hexagonal sheet barium ferrite
A technology of hexagonal flakes and barium ferrite, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of reducing the utilization rate of raw materials, environmental pollution, etc., and achieve improved utilization rate, uniform heating, and saving raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment provides a preparation process for preparing high-purity hexagonal flake barium ferrite by a chemical co-precipitation method, and the preparation steps are as follows:
[0036] (1) Prepare BaCl with a concentration of 0.5mol / L 2 Solution and 1mol / L FeCl 3 The solution is mixed with a barium-iron stoichiometric ratio of 1:10 and stirred evenly; the stirring speed is 600 rpm;
[0037] (2) Prepare a NaOH solution with a concentration of 6mol / L, and instill BaCl through a basic burette at a titration rate of 1 drop per second 2 And FeCl 3 In the mixture, BaCl 2 The stoichiometric ratio to the added NaOH is 1:38;
[0038] (3) During the dropping process, use a magnetic stirrer to continuously stir to make the reaction uniform, and let it stand for 10 minutes after the reaction is completed; the stirring speed is 600 rpm;
[0039] (4) The precursor obtained by the reaction after mixing is suction filtered, dried, and then heated to 1000°C at a heating rate of 5°C / min ...
Embodiment 2
[0046] This embodiment provides a preparation process for preparing high-purity hexagonal flake barium ferrite by a chemical co-precipitation method, and the preparation steps are as follows:
[0047] (1) Prepare BaCl with a concentration of 0.5mol / L 2 Solution and 1mol / L FeCl 3 The solution is mixed with a barium-iron stoichiometric ratio of 1:9 and stirred evenly; the stirring speed is 600 rpm;
[0048] (2) Prepare a NaOH solution with a concentration of 6mol / L, and instill BaCl through a basic burette at a titration rate of 1 drop per second 2 And FeCl 3 In the mixture, BaCl 2 The stoichiometric ratio to the added NaOH is 1:38;
[0049] (3) During the dropping process, use a magnetic stirrer to continuously stir to make the reaction uniform, and let it stand for 10 minutes after the reaction is completed; the stirring speed is 600 rpm;
[0050] (4) The precursor obtained by the reaction after mixing is suction filtered, dried, and then heated to 800°C at a heating rate of 5°C / min fo...
Embodiment 3
[0053] This embodiment provides a preparation process for preparing high-purity hexagonal flake barium ferrite by a chemical co-precipitation method, and the preparation steps are as follows:
[0054] (1) Prepare BaCl with a concentration of 0.5mol / L 2 Solution and 1mol / L FeCl 3 The solution is mixed with a barium-iron stoichiometric ratio of 1:11 and stirred evenly; the stirring speed is 600 rpm;
[0055] (2) Prepare a NaOH solution with a concentration of 6mol / L, and instill BaCl through a basic burette at a titration rate of 1 drop per second 2 And FeCl 3 In the mixture, BaCl 2 The stoichiometric ratio to the added NaOH is 1:38;
[0056] (3) During the dropping process, use a magnetic stirrer to continuously stir to make the reaction uniform, and let it stand for 10 minutes after the reaction is completed; the stirring speed is 600 rpm;
[0057] (4) The precursor obtained by the reaction after mixing is suction filtered, dried, and then heated to 800°C at a heating rate of 5°C / min f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com