Copper current collector for lithium metal secondary battery, preparation method thereof and lithium metal secondary battery
A secondary battery, lithium metal technology, applied in the field of lithium metal secondary battery, copper current collector for lithium metal secondary battery, can solve problems such as unfavorable environmental safety, unfavorable large-scale production, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a method for preparing a copper current collector for a lithium metal secondary battery, comprising the following steps:
[0042] Using Tris-HCl buffer solution as a solvent, adding a modifier to prepare a modified solution;
[0043] placing the copper foil in the modified solution, and shaking at a constant temperature of 20-60° C. for 12-50 hours to obtain a copper current collector for a lithium metal secondary battery;
[0044] The modifying agent is a functional polymer and inorganic nanoparticles;
[0045] The functional polymer is a functional polymer with one or more groups in cyano group, hydroxyl group, amino group, aldehyde group and carboxyl group;
[0046] The inorganic nanoparticles are one or more of alkylated alumina nanoparticles, nano silicon dioxide, nano calcium carbonate, nano calcium phosphate and iron oxide nanoparticles;
[0047] In the present invention, the surface of the copper foil is preferably cleaned w...
Embodiment 1
[0058] Wash the surface of Cu foil with deionized water to remove impurities on the surface of Cu foil.
[0059] Prepare 0.01M Tris solution and 0.01M HCl solution respectively. Slowly drop the prepared HCl solution into the Tris solution to adjust the pH value of the solution to obtain a Tris-HCl buffer solution with a pH value of 8.5. With the above-mentioned buffer solution as a solvent, the catechol (CA) buffer solution of 2.5 g / L is prepared with the above-mentioned buffer solution as a solvent;
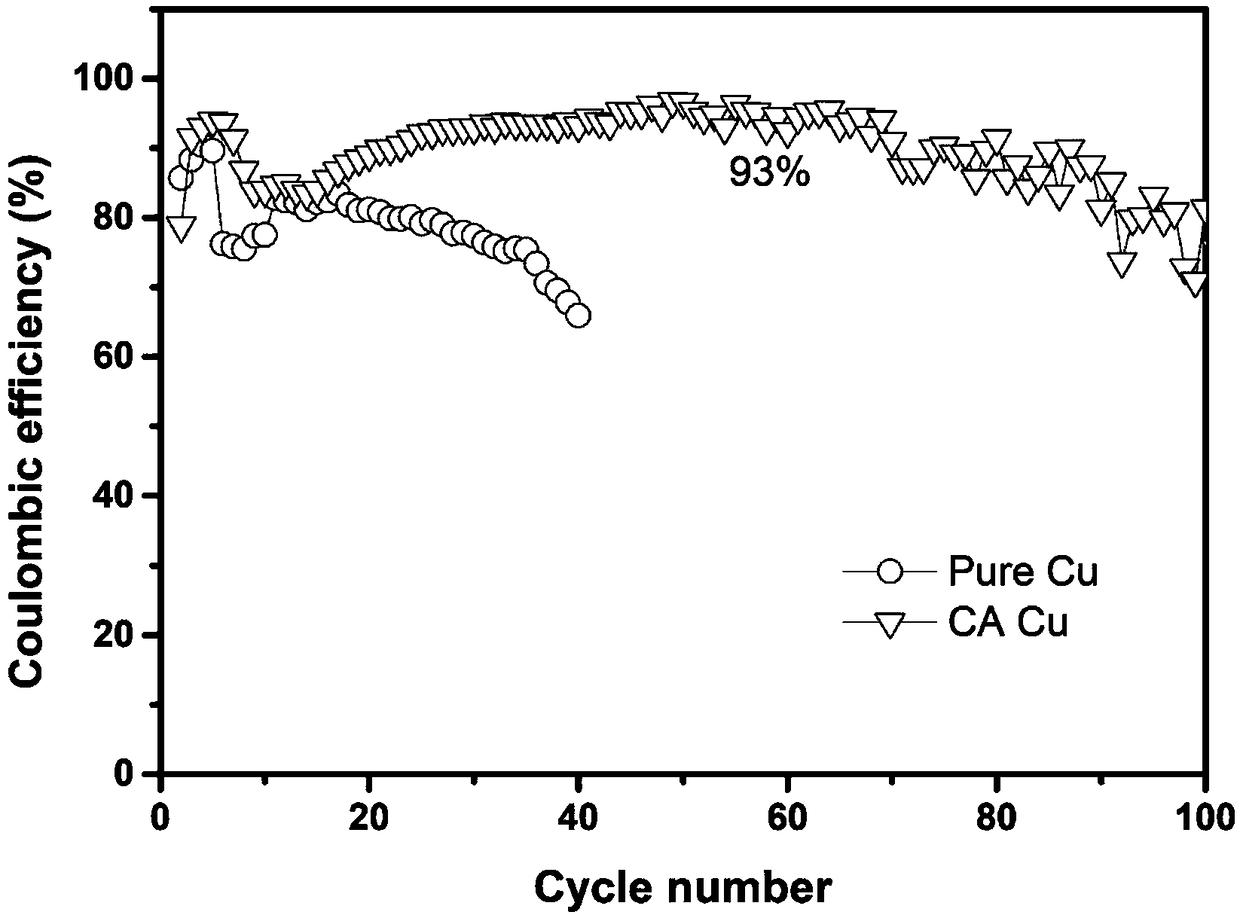
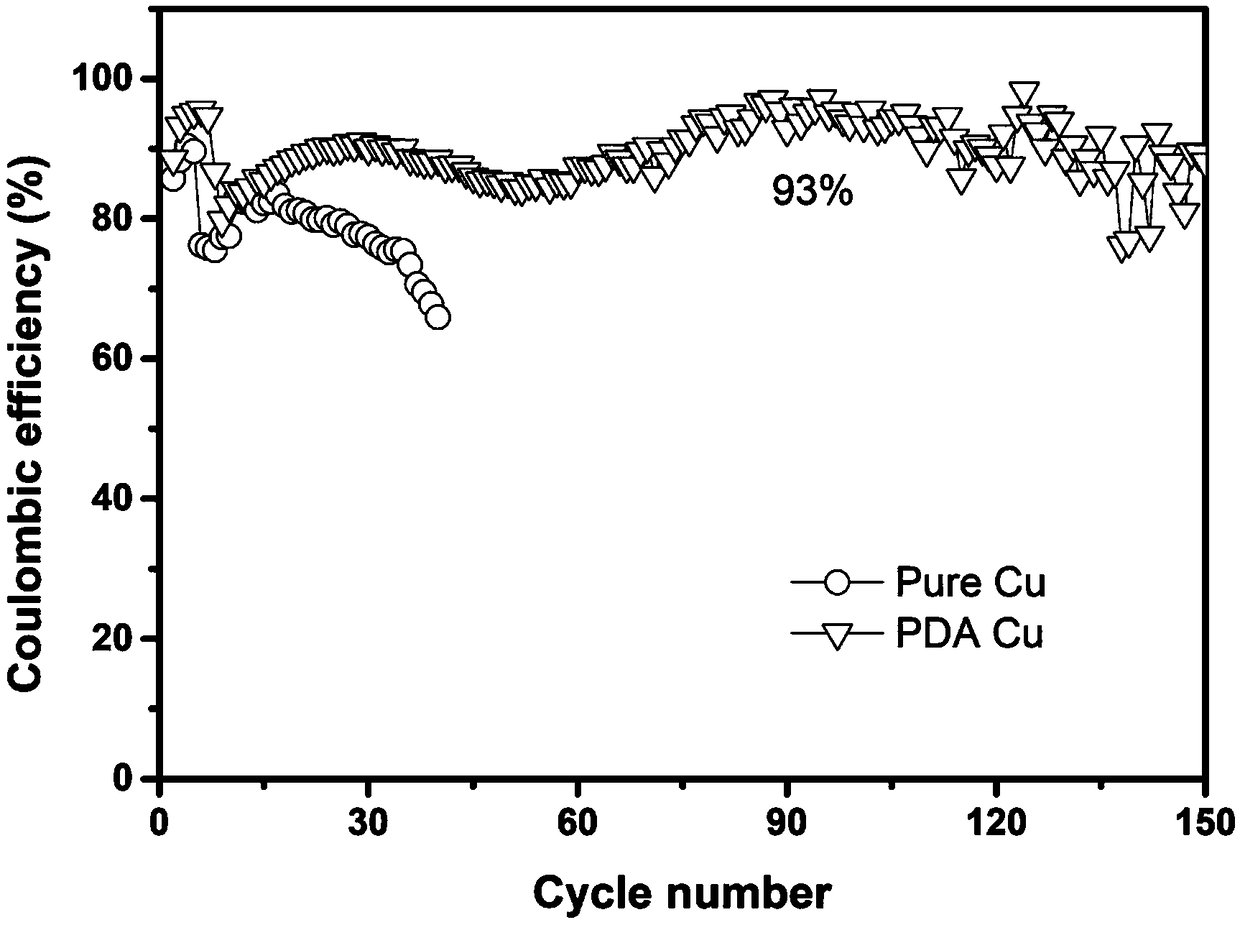
[0060] The copper foil was put into a catechol (CA) buffer solution, and was shaken at a constant temperature of 50°C for 24 hours so that the generated polymer was evenly introduced to the surface of the copper foil, thereby preparing a functional polymer layer modified copper current collector.
[0061] The copper current collector is used to prepare a lithium metal half-cell, and the specific composition is as follows:
[0062] The functional polymer layer modified copper c...
Embodiment 2
[0065] Wash the surface of Cu foil with deionized water to remove impurities on the surface of Cu foil.
[0066] Prepare 0.01M Tris solution and 0.01M HCl solution respectively. Slowly drop the prepared HCl solution into the Tris solution to adjust the pH value of the solution to obtain a Tris-HCl buffer solution with a pH value of 8.5. With the above-mentioned buffer solution as a solvent, the norepinephrine (NA) buffer solution of 2.5 g / L is prepared with the above-mentioned buffer solution as a solvent;
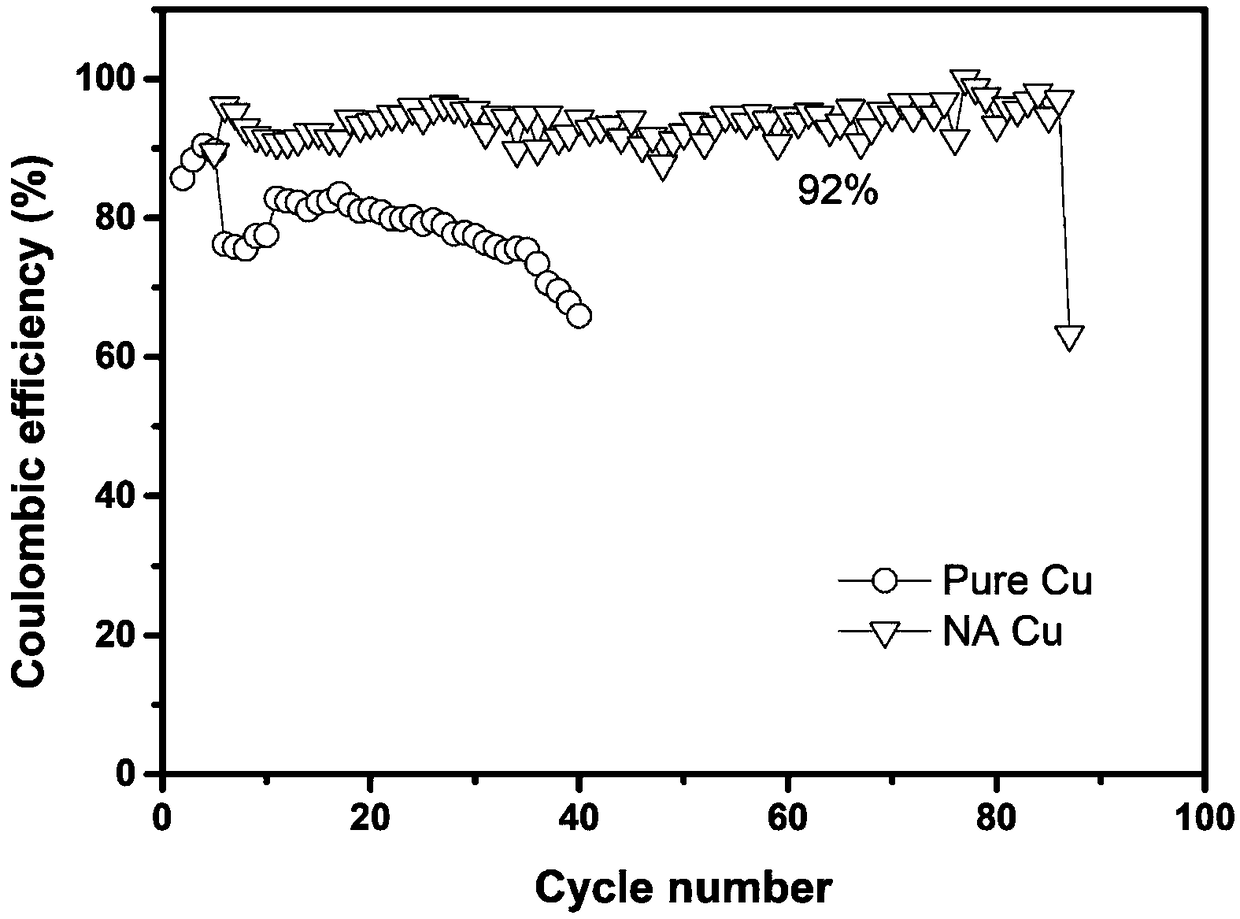
[0067] The copper foil was put into a norepinephrine (NA) buffer solution, and was shaken at a constant temperature of 50°C for 24 hours so that the generated polymer was evenly introduced to the surface of the copper foil, thereby preparing a functional polymer layer modified copper current collector.
[0068] The obtained curve is as figure 2 As shown, it can be seen that the coulombic efficiency of the functional polymer layer modified copper current collector is 92%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com