Recombinant Escherichia coli capable of realizing soluble expression of linoleate isomerase and application of recombinant Escherichia coli
A technology of recombinant Escherichia coli and linoleic acid isomerase, which is applied in the field of bioengineering and can solve the problems of low soluble expression level and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Construction method of recombinant Escherichia coli BL21 (DE3) (pET24a-Mpai)
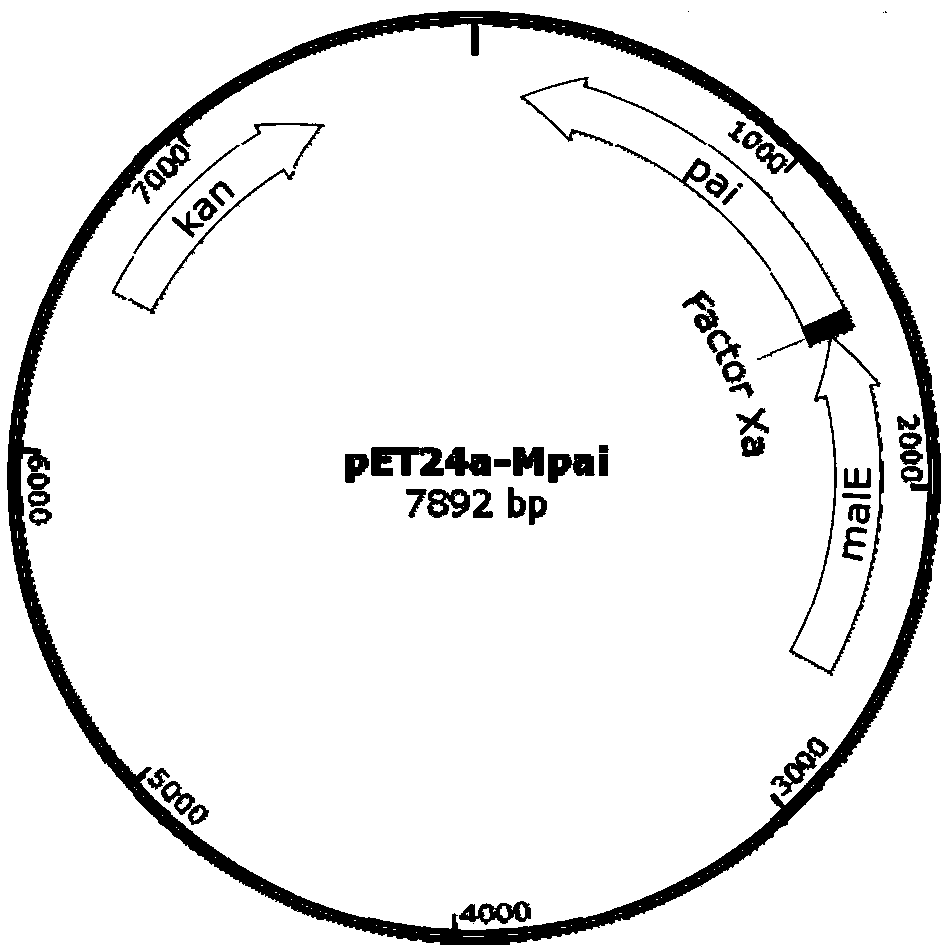
[0030]Using the genome of Escherichia coli K12 (NC_000913.3) containing the gene malE of the maltose-binding protein MBP as a template, the whole malE gene was synthesized and connected to the plasmid pUC57, and pUC57- malE, excise the malE gene and recover by cutting the gel, and the same expression vector pET24a-pai containing the pai gene (Genbank: AX062088.1) (for the construction method, refer to "Linoleic acid isomerase gene in oleaginous fungi) Heterologous expression and biosynthesis of products" (Zhang Baixi)); after ligation reaction at 16°C overnight, the malE gene was inserted into the upstream of the pai gene of the corresponding expression vector, and the fusion protein expression vector pET24a-Mpai was constructed ( figure 1 ). Transform E.coli BL21 (DE3) competent and spread on LB plates containing 50 μg / mL kanamycin, and identify the positive clones after trans...
Embodiment 2
[0031] Embodiment 2: Product analysis of recombinant Escherichia coli BL21 (DE3) (pET24a-Mpai)
[0032] Pick a single colony of the recombinant strain obtained in Example 1, inoculate it in 5 mL of LB medium containing 50 μg / mL kanamycin, cultivate overnight at 37° C. with shaking at 200 r / min, and then inoculate it at a ratio of 2% (v / v). Transfer to 50mL LB medium containing 50μg / mL kanamycin, culture at 37°C, 200r / min shaking for 3-4h to OD 600 The value is 0.6-0.8, add IPTG to a final concentration of 1mmol / L, and culture with shaking at 20°C for 24h.
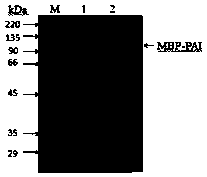
[0033] Collect the bacteria by centrifugation at 4°C and 10,000r / min, and resuspend them in 100mmol / L Tris-HCl buffer (pH7.4), sonicate the bacteria and centrifuge at 4°C and 6,000r / min for 10min, and collect the supernatant It is the whole-cell protein, take 10 μL whole-cell protein, add 10 μL 1× protein loading buffer, pipette and beat to mix evenly, then bathe in boiling water for 10 min, and use SDS-PAGE electrophoresi...
Embodiment 3
[0035] Example 3: Optimization of expression conditions induced by recombinant Escherichia coli BL21 (DE3) (pET24a-Mpai)
[0036] With the Escherichia coli BL21 (DE3) (pET24a-Mpai) that embodiment 1 obtains as object, with the LB culture medium of embodiment 2 as the basis, consider the temperature of induced expression, inducer concentration and induction time to fusion protein MBP-PAI effect to further increase the expression level.
[0037] (1) Induction temperature optimization
[0038] Induction temperature is very important to the growth of bacteria and protein expression. In order to explore the optimal induction temperature, the recombinant protein MBP-PAI was expressed under different induction temperature conditions.
[0039] Pick a single bacterium colony of the recombinant strain obtained in Example 1, inoculate in 5 mL of LB medium containing 50 μg / mL kanamycin, cultivate overnight at 37° C., and transfer to 50 mL of In LB medium containing 50 μg / mL kanamycin, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com