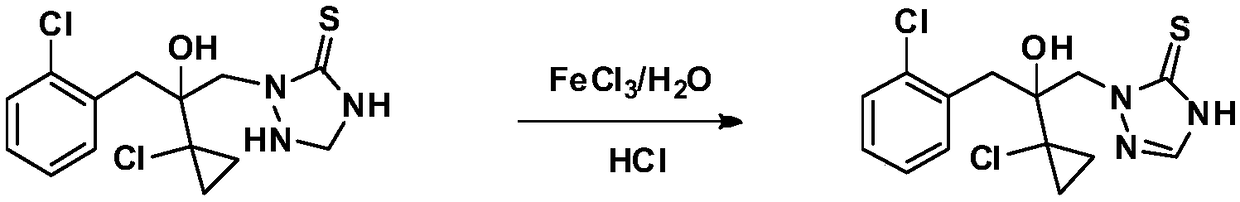
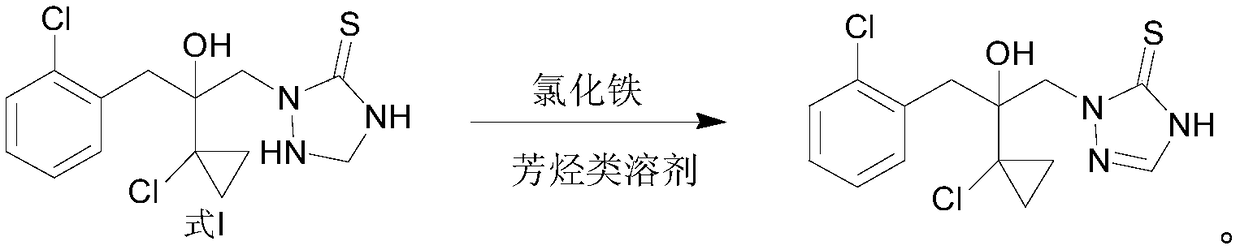
Preparation method of prothioconazole
A technology of prothioconazole and heterogeneous oxidation, applied in the direction of organic chemistry, etc., can solve the problems of large amount of solvent, low product yield, unreusable oxidant, etc., and achieves easy large-scale production and realizes recycling. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] At room temperature, 10.0 g (99.4% purity, 28.7 mmol) of 2-(1-chloro-cyclopropan-1-yl)-1-(2-chloro-phenyl)-3-(4,5-bis Hydrogen-1,2,4-triazolidine-5-thiocarbon-1-yl)-propan-2-ol, 30g toluene and 35.0g (66.0mmol) ferric chloride aqueous solution were added to a 250mL four-necked flask and stirred evenly , and then the temperature was raised to between 30-40° C., and the reaction progress was monitored by HPLC. After the reaction was completed, the temperature was lowered to 10-20° C., and 8.5 g of solids were obtained after suction filtration and drying. HPLC analysis showed that the solid contained 98.1% of 2-(1-chloro-cyclopropan-1-yl)-1-(2-chloro-phenyl)-3-(4,5-dihydro-1,2, 4-triazol-5-thiocarbon-1-yl)-propan-2-ol. Therefore, the calculated yield is 83.9% of the theoretical value (this yield is the yield after the first batch of reactions, and after the organic phase is reused, the yield is 99%). The filtrate was separated into layers to obtain 22.5 g of an organic ...
Embodiment 2
[0040]At room temperature, 10.0 g (99.4% purity, 28.7 mmol) of 2-(1-chloro-cyclopropan-1-yl)-1-(2-chloro-phenyl)-3-(4,5-bis Hydrogen-1,2,4-triazolidine-5-thiocarbon-1-yl)-propan-2-ol, 22.5g of the organic phase recovered in Example 1, 7.5g of fresh toluene and 35.0g (66.0 mmol) the aforementioned regenerated ferric chloride aqueous solution was added into a 250mL four-neck flask and stirred evenly, then the temperature was raised to 30-40°C, and the reaction progress was monitored by HPLC. After the reaction was completed, the temperature was lowered to 10-20° C., suction filtered and dried to obtain 9.94 g of solid. HPLC analysis showed that the solid contained 98.5% of 2-(1-chloro-cyclopropan-1-yl)-1-(2-chloro-phenyl)-3-(4,5-dihydro-1,2, 4-triazol-5-thiocarbon-1-yl)-propan-2-ol. Therefore, the calculated yield was 99.0% of theory. The filtrate was separated into layers to obtain 23.0 g of an organic phase and 35.0 g of an aqueous phase. The organic phase can be used dire...
Embodiment 3
[0043] At room temperature, 10.0 g (99.4% purity, 28.7 mmol) of 2-(1-chloro-cyclopropan-1-yl)-1-(2-chloro-phenyl)-3-(4,5-bis Hydrogen-1,2,4-triazolidine-5-thiocarbon-1-yl)-propan-2-ol, 25.0g chlorobenzene and 35.0g (66.0mmol) ferric chloride aqueous solution were added to a 250mL four-necked flask Stir evenly, then heat up to 30-40°C, and monitor the reaction progress by HPLC. After the reaction was completed, the temperature was lowered to 10-20° C., and 8.7 g of solids were obtained after suction filtration and drying. HPLC analysis showed that the solid contained 98.0% of 2-(1-chloro-cyclopropan-1-yl)-1-(2-chloro-phenyl)-3-(4,5-dihydro-1,2, 4-triazol-5-thiocarbon-1-yl)-propan-2-ol. Therefore, the calculated yield was 86.3% of theory. The filtrate was separated into layers to obtain 22.0 g of an organic phase and 34.5 g of an aqueous phase. The organic phase can be used directly as a solvent for the next batch of oxidation reactions.
[0044] A small amount of concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com