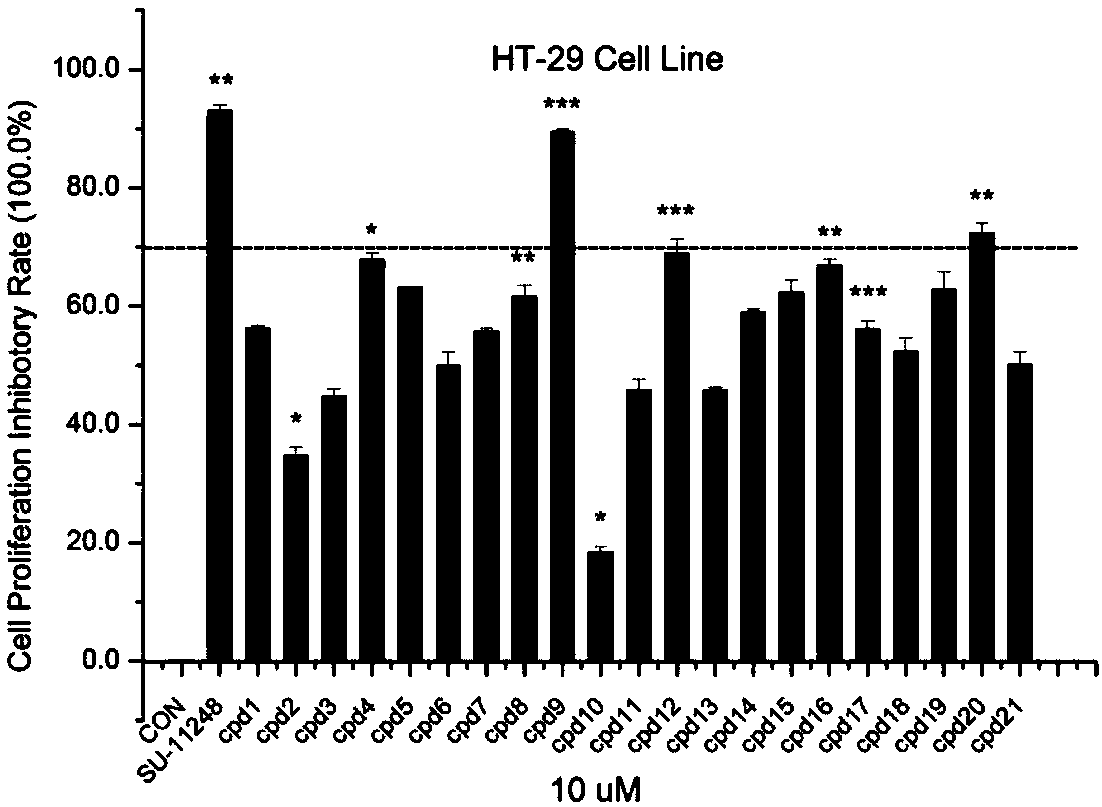
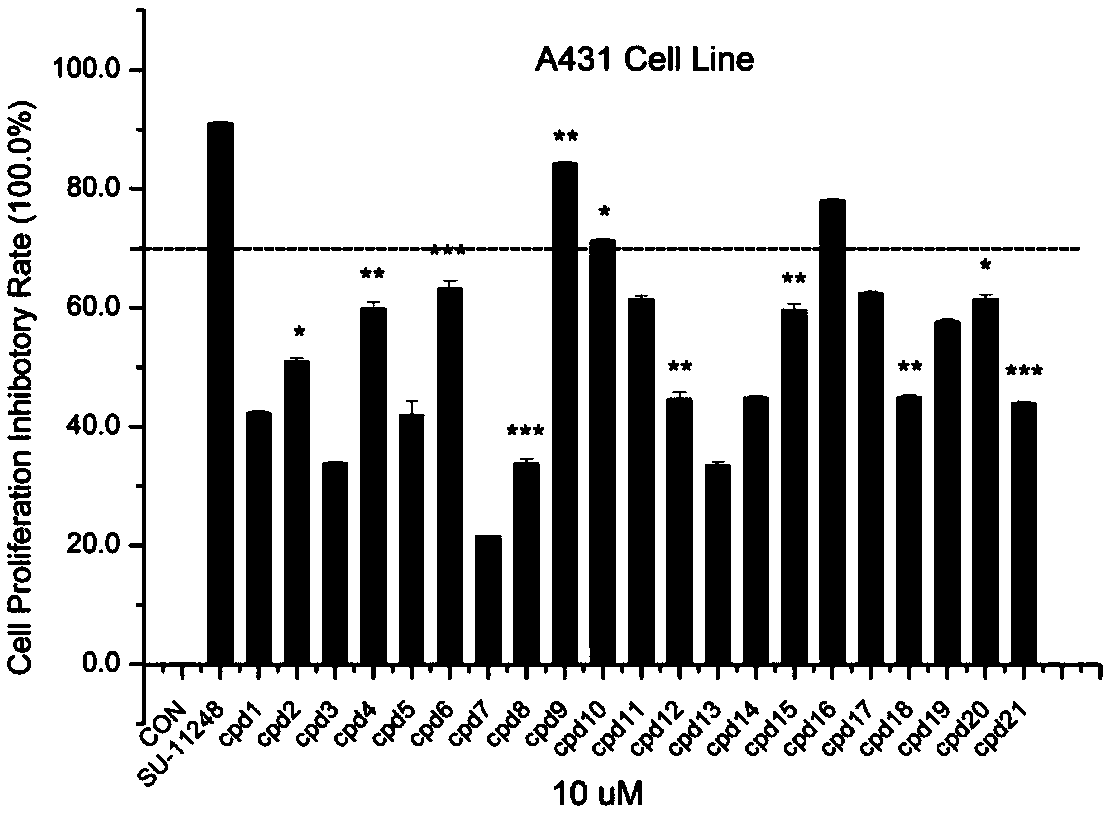
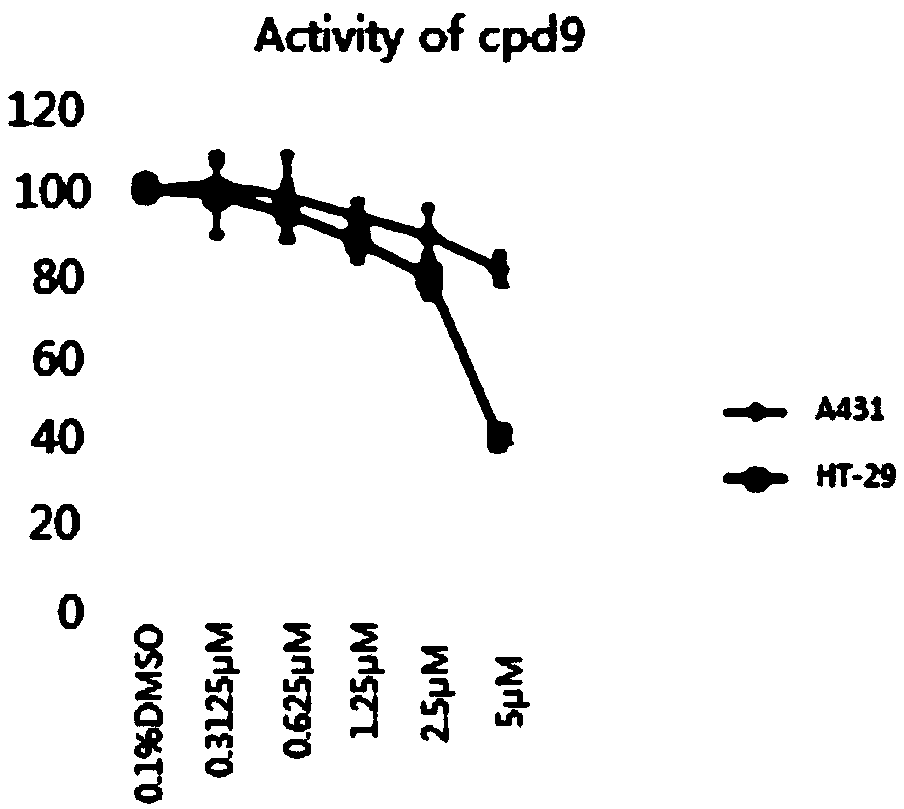
Compound for treating tumor
A technology of compound and solvate, which is applied in the field of drug synthesis, can solve the problems such as few complete cures, achieve excellent anti-tumor activity, excellent application prospects, and the effect of inhibiting new blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The synthesis of embodiment 1, compound 1
[0057] synthetic route:
[0058]
[0059] Preparation:
[0060] (1) Add compound a (10g, 1.0eq) and dichloromethane (100ml) into a three-necked flask, under nitrogen protection, cool down to -78°C, add boron tribromide (10eq) in dichloromethane solution (1g / 3ml). After the addition, keep the reaction for 1 hour, heat up to 25°C and react for 4-6 hours. After the reaction, wash with 10ml of water, dry with anhydrous sodium sulfate, concentrate the organic phase to dryness, and pass through a flash column to obtain 6.97g of compound b. The yield is: 80.5%.
[0061] (2) Compound b (5g, 1.0eq) and pyridine (50ml) were added to a three-necked flask, and under nitrogen protection, acetic anhydride (2.5eq) was added while the temperature was controlled below 20°C. After the addition is complete, heat the reaction for 1 hour, raise the temperature to 25°C and react for 4-6 hours. After the reaction, pour the reaction liquid in...
Embodiment 2
[0069] The synthesis of embodiment 2, compound 2
[0070]
[0071] The benzoyl chloride in step (7) of Example 1 is replaced by 2-fluorobenzoyl chloride, and the same preparation method as in Example 1 can be used to prepare compound 2, 1 H NMR (300MHz, CDCl 3 , 25°C, TMS): δ=2.13-2.38(m, 4H, CH2), 3.29-3.39(m, 4H, CH2), 4.0(s, 1H, NH), 6.79(s, 1H, CH), 7.16( s,1H,CH)7.24(s,1H,CH)7.29(s,1H,CH),7.40(s,1H,CH).7.41(s,1H,CH)7.42(s,1H,CH),8.00 (s, 1H, CH), 8.01 (s, 1H, CH) 8.49 (s, 1H, CH). Molecular formula: C 26 h 19 CIF 2 N 4 o 3 , relative molecular mass: 508.9.
Embodiment 3
[0072] The synthesis of embodiment 3, compound 3
[0073]
[0074] By replacing p-fluorobenzoyl chloride with benzoyl chloride in step (7) of Example 1, and using the same preparation method as in Example 1, compound 3 can be prepared, 1 H NMR (300MHz, CDCl 3 , 25°C, TMS): δ=2.13-2.38(m, 4H, CH2), 3.29-3.39(m, 4H, CH2), 4.0(s, 1H, NH), 6.79(s, 1H, CH), 7.16( s,1H,CH)7.24(s,1H,CH),7.29(s,1H,CH),7.41(s,1H,CH),7.42(s,2H,CH),.8.12(s,2H,CH ), 8.49 (s, 1H, CH). Molecular formula: C 26 h 19 CIF 2 N 4 o 3 , relative molecular mass: 508.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com