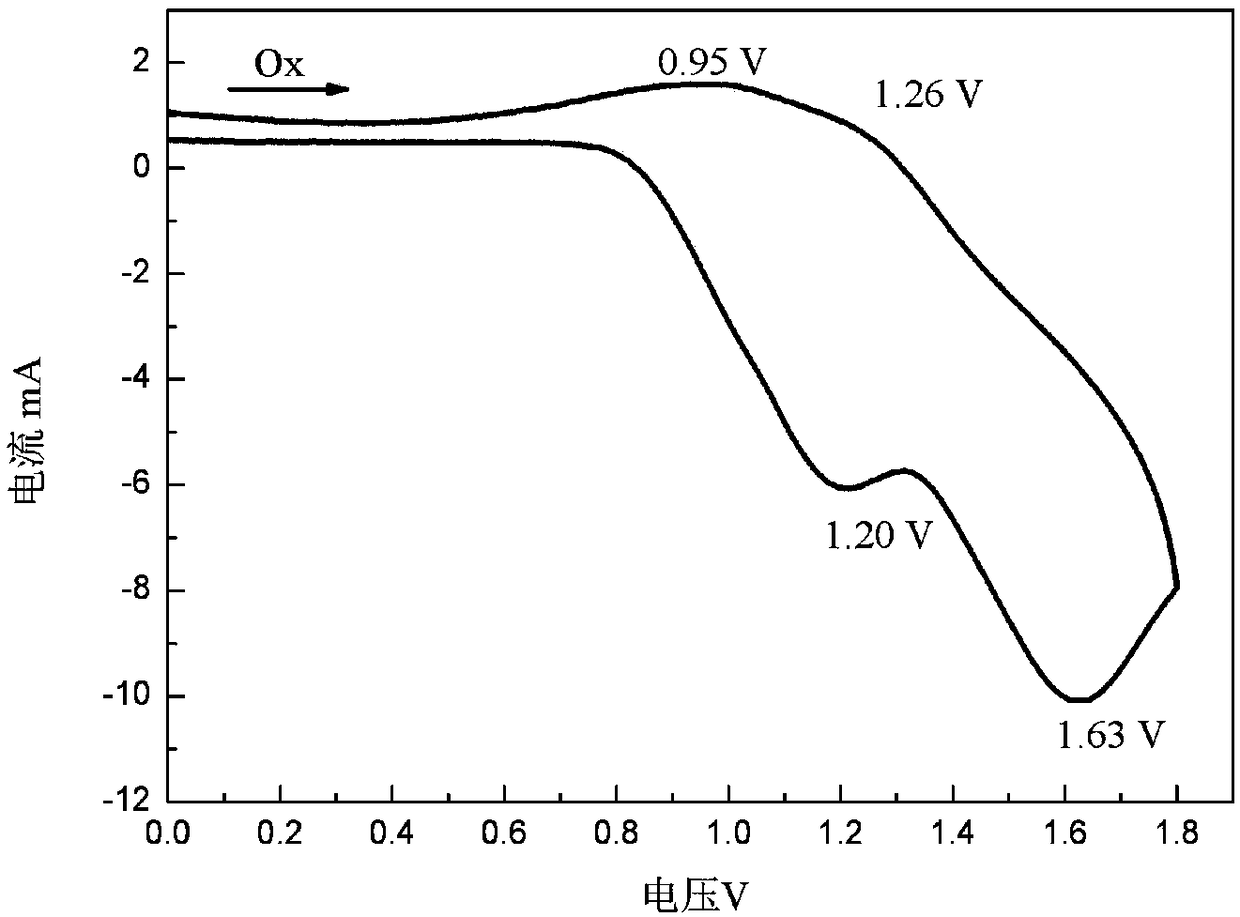
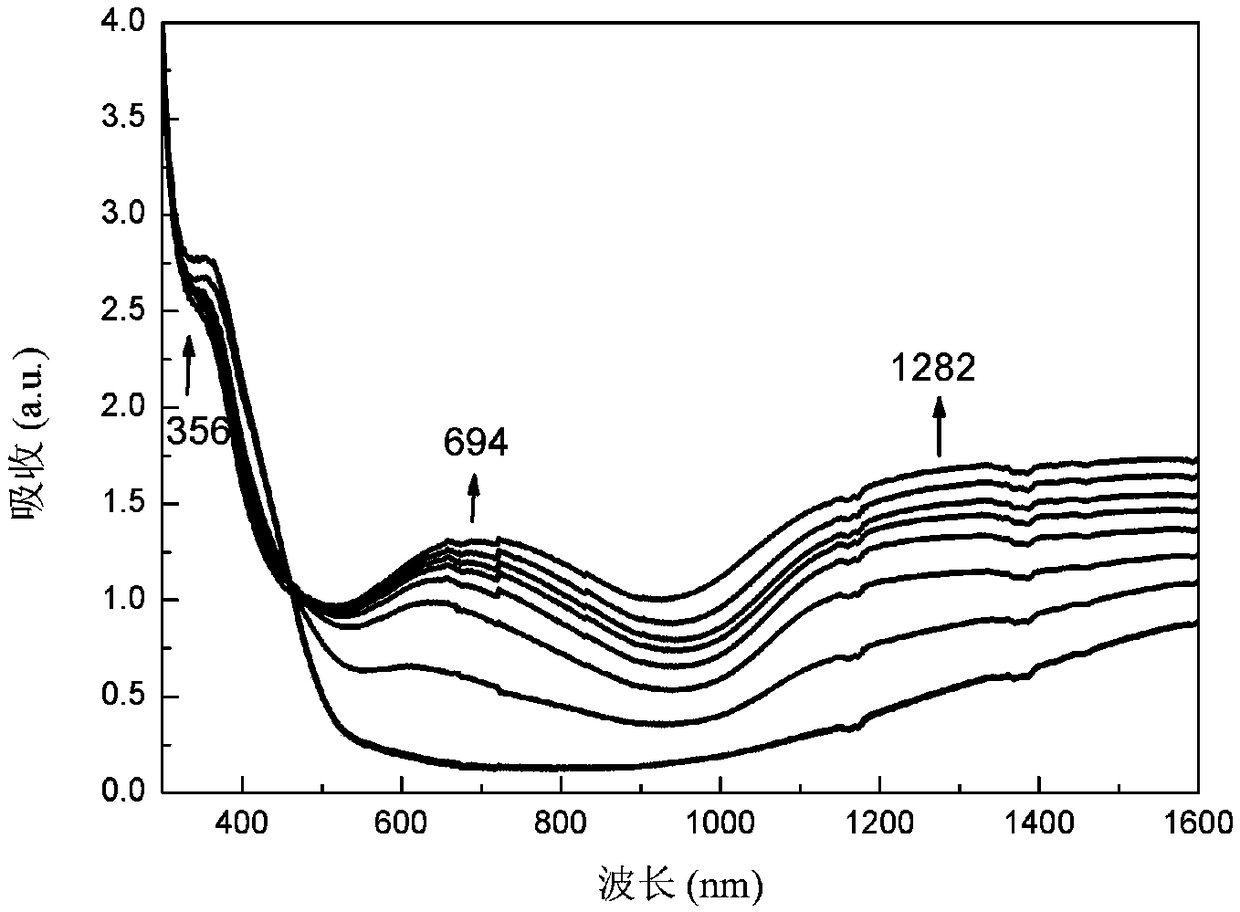
DPP (diketopyrrolopyrrole) block polymer with carbazole groups, method for preparing DPP block polymer and application thereof
A technology of block polymers and carbazole groups, applied in the field of DPP block polymers, can solve the problems of low solubility, difficult processing and film formation, etc., and achieve the effects of improving solubility, excellent electrochromic and memory properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
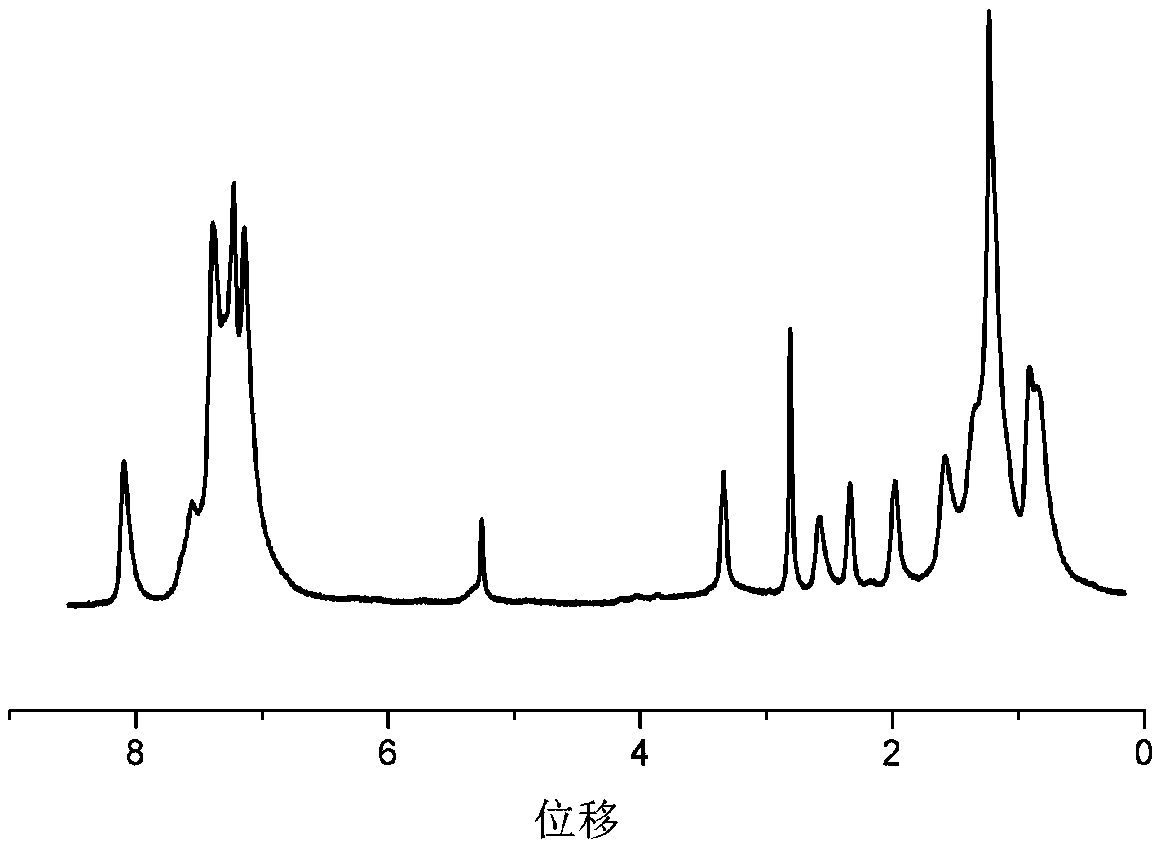
[0024] Specific embodiment one: the structural formula of the DPP block polymer containing carbazole group in this embodiment is as follows:
[0025] ,
[0026] In the formula, n is a positive integer.
specific Embodiment approach 2
[0027] Embodiment 2: This embodiment differs from Embodiment 1 in that: in the structural formula of the DPP block polymer containing carbazole groups, n is a positive integer ranging from 3 to 10. It is the same as the first embodiment.
specific Embodiment approach 3
[0028] Specific embodiment three: the preparation method of the DPP block polymer containing carbazole group in this embodiment is carried out according to the following steps:
[0029] 1. Synthesis of 3,6-bis(thiophen-2-yl)-2,5-bis(8-(tributylstannyl)octyl)pyrrolo[3,4-c]pyrrole-1,4(2H, 5H)-diketone: ①, mix tert-amyl alcohol and sodium, add 2-cyanothiophene and dimethyl succinate to it, pass into N 2 For protection, react at a temperature of 90-120°C for 18-28 hours, then lower the reaction temperature from 90-120°C to 60-80°C, then add glacial acetic acid drop by drop until the reaction liquid is viscous with solid precipitation, and then Add methanol and water to dilute, then cool to room temperature under stirring, wash with water for 3 to 5 times and then with methanol for 3 to 5 times to obtain a deep red solid, which is DPP; ②, the DPP obtained in step ①, Potassium tert-butoxide and anhydrous DMF were mixed, and N 2 For protection, react at a temperature of 100°C for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com