Double-perovskite catalyst as well as preparation method and application thereof
A double perovskite and catalyst technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, heterogeneous catalyst chemical elements, etc., can solve the problems of low specific surface area, limited perovskite, poor characteristics, etc., to achieve High catalytic activity, excellent thermal stability, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
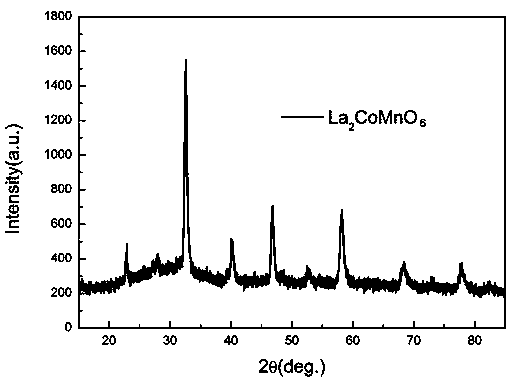
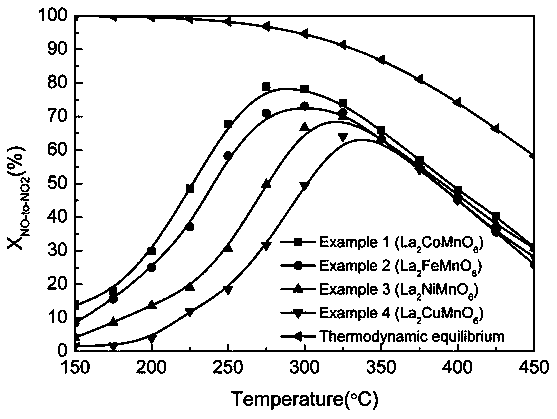
[0022] Embodiment 1: The double perovskite catalyst of this embodiment is La 2 CoMnO 6 ;
[0023] A kind of preparation method of double perovskite catalyst, concrete steps are as follows:
[0024] (1) Add salt A (salt A is lanthanum nitrate), salt B (salt B is cobalt nitrate) and salt B' (salt B' is manganese nitrate) into absolute ethanol and grind until dissolved to obtain ethanol metal salt solution, The molar ratio of A salt (lanthanum nitrate), B salt (cobalt nitrate) and B' salt (manganese nitrate) is 2:1:1;
[0025] (2) Mix the ethanol metal salt solution, potassium nitrate and sodium nitrate obtained in step (1), dry and volatilize the ethanol to obtain a mixed salt; wherein A salt (lanthanum nitrate), B salt (cobalt nitrate) and B' salt (manganese nitrate ) to the total molar ratio of potassium nitrate and sodium nitrate is 1:30; the molar ratio of potassium nitrate and sodium nitrate is 1:1;
[0026] (3) The mixed salt obtained in step (2) was heated to 700°C at...
Embodiment 2
[0030] Embodiment 2: The present embodiment double perovskite catalyst is La 2 FeMnO 6 ;
[0031] A kind of preparation method of double perovskite catalyst, concrete steps are as follows:
[0032] (1) Add salt A (salt A is lanthanum nitrate), salt B (salt B is iron nitrate) and salt B' (salt B' is manganese nitrate) into absolute ethanol and grind until dissolved to obtain ethanol metal salt solution, The molar ratio of A salt (lanthanum nitrate), B salt (iron nitrate) and B' salt (manganese nitrate) is 2:1:1;
[0033] (2) Mix the ethanol metal salt solution, potassium nitrate and sodium nitrate obtained in step (1), dry and volatilize the ethanol to obtain a mixed salt; wherein A salt (lanthanum nitrate), B salt (iron nitrate) and B' salt (manganese nitrate ) to the total molar ratio of potassium nitrate and sodium nitrate is 1:30; the molar ratio of potassium nitrate and sodium nitrate is 1:1.4;
[0034] (3) The mixed salt obtained in step (2) was heated to 800°C at a c...
Embodiment 3
[0037] Embodiment 3: The double perovskite catalyst of this embodiment is La 2 NiMnO 6 ;
[0038] A kind of preparation method of double perovskite catalyst, concrete steps are as follows:
[0039] (1) Add salt A (salt A is lanthanum nitrate), salt B (salt B is nickel nitrate) and salt B' (salt B' is manganese nitrate) into absolute ethanol and grind until dissolved to obtain ethanol metal salt solution, The molar ratio of A salt (lanthanum nitrate), B salt (nickel nitrate) and B' salt (manganese nitrate) is 2:1:1;
[0040] (2) Mix the ethanol metal salt solution, potassium nitrate and sodium nitrate obtained in step (1), dry and volatilize the ethanol to obtain a mixed salt; wherein A salt (lanthanum nitrate), B salt (nickel nitrate) and B' salt (manganese nitrate ) to the total molar ratio of potassium nitrate and sodium nitrate is 1:20; the molar ratio of potassium nitrate and sodium nitrate is 1:1.5;
[0041] (3) The mixed salt obtained in step (2) was heated to 800°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com