Biotin-labeled ebselen probe, and preparation method and application of probe
A biotin-labeled and ebselen technology is applied in the field of biotin-labeled ebselen probes and the preparation thereof, which can solve the problems of inability to accurately explore ebselen proteins and the like, and achieves fast labeling and preparation. Simple method and precise structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
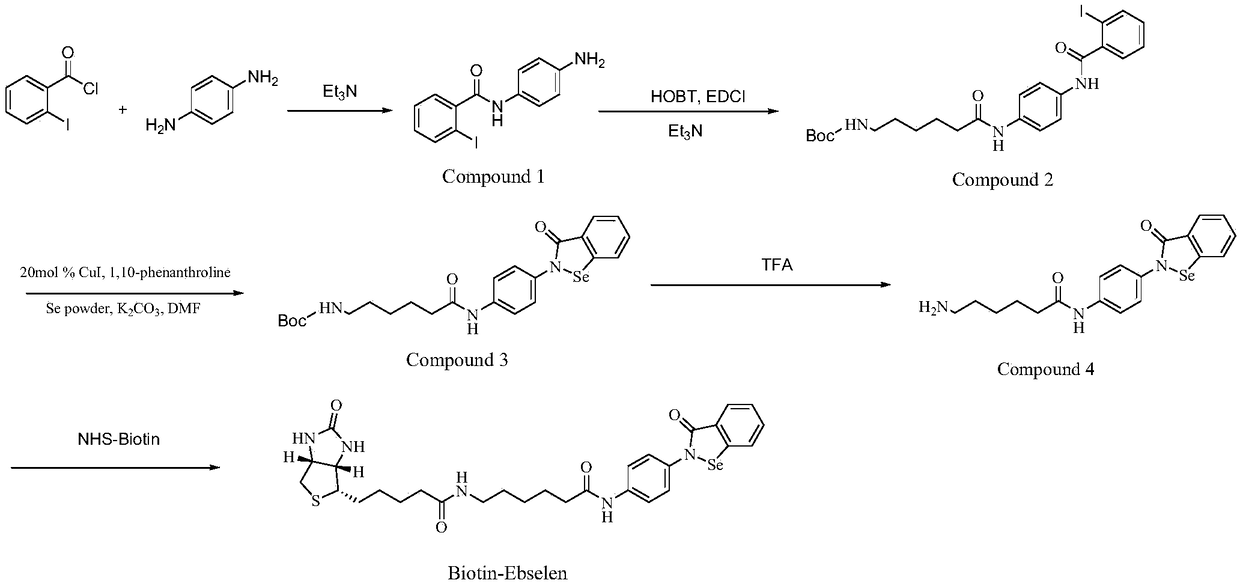
[0046]Embodiment A kind of preparation method of biotin-labeled ebselen probe
[0047] The preparation method of the biotin-labeled ebselen probe comprises the following steps:
[0048] (1) O-iodobenzoyl chloride (1.33g) and p-phenylenediamine (1.08g) were dissolved in CH 2 Cl 2 (10 mL each), and triethylamine (1.04 mL) was added to the p-phenylenediamine solution, and then the o-iodobenzoyl chloride solution was added dropwise to the p-phenylenediamine solution in an ice bath, and reacted overnight at room temperature , the reaction was washed with water, and after separation, the aqueous phase was washed with CH 2 Cl 2 Carry out extraction twice, organic phase merges, after drying, spin dry, separate with chromatographic column (eluent: V 石油醚 :V 二氯甲烷 =40:1), the obtained solid is compound I.
[0049] ESI-MS: calculated for [M+H] + =338.9988,found338.9969.
[0050] (2) Dissolve tert-butoxycarbonyl 6-aminocaproic acid (1.02g), EDCI·HCl (0.93g) and HOBT (0.65g) in CH 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com