A clathrate of an artemisinin type medicine and ring-opened cucurbituril and a preparation method thereof
A technology of artemisinin and cucurbitacil, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problem of cyclodextrin and its derivatives not being resistant to strong acid and strong alkali, low bioavailability, and containing The problem of compound instability and other problems can be overcome, and the effect of overcoming low bioavailability, improving solubility, and simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthetic preparation of tetramer
[0026] Carry out with reference to the method in literature Ma D, et al.Nature Chemistry, 2012,4(6):503:
[0027] (1) Synthesis of dimer: mix glycoluril (500g, 3.51mol) and paraformaldehyde (105g, 3.51mol) evenly, add to HCl solution (8M, 700mL), and heat the reaction solution at 50°C for 48h; After the reaction, the reaction solution was cooled and vacuum filtered to obtain the crude product; the crude product was washed with water (500 mL), and then recrystallized with TFA (1.5 L) to obtain a dimer as a white solid (334 g, 62%) ;
[0028]
[0029] (2) Synthesis of tetramer: dimer (304g, 1.20mol) was added to anhydrous MeSO 3 In H (600mL), after the dimer is completely dissolved, the solution becomes transparent, then etherified methyl glycoluril (84g, 0.27mol) is added; the reaction solution is reacted at 50°C for 3h; The solution was poured into water (6.0L); after vacuum filtration, the crude product was dr...
Embodiment 2
[0037] Example 2: Preparation of inclusion compound of dihydroartemisinin and two kinds of open-ring cucurbituril
[0038] (1) Preparation of inclusion compound of dihydroartemisinin and secocucurbituril 1
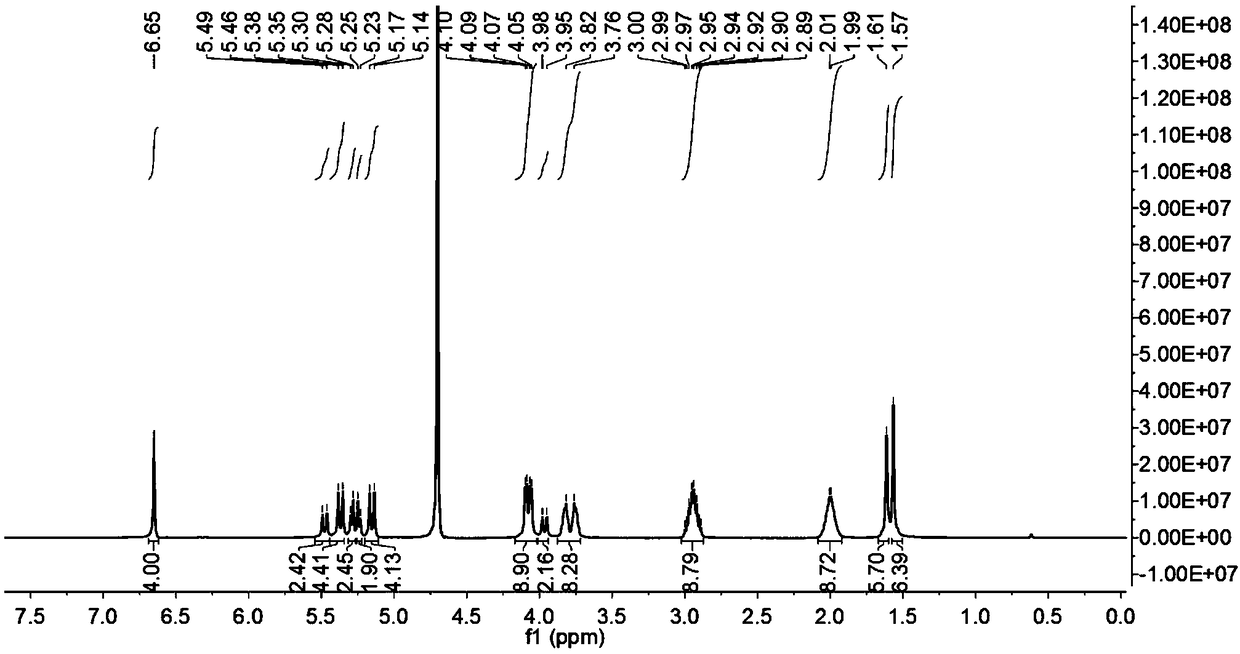
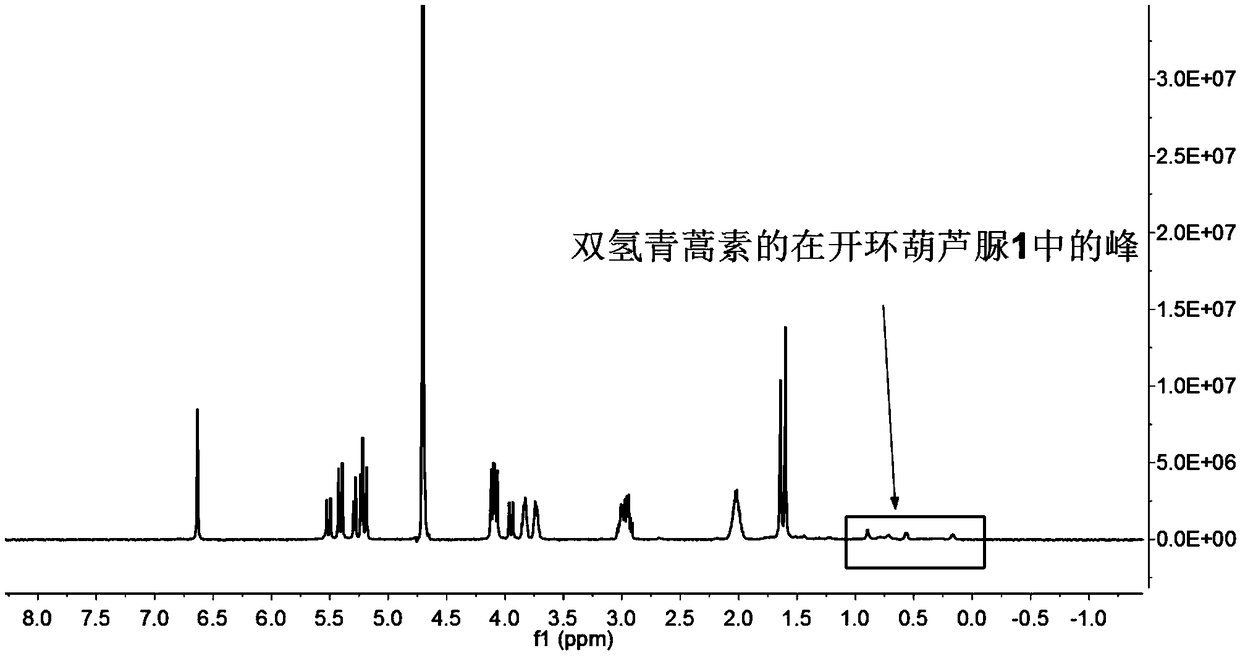
[0039] Weigh 0.172g (0.1mmol) of ring-opening cucurbituril and dissolve it in 15mL of distilled water, stir until dissolved at 25°C, dissolve 0.116g (0.3mmol) of dihydroartemisinin in 10mL of ethanol solution, and Mix the two solutions under the same conditions, and stir for 72 hours in the dark, then filter with filter paper and microporous membrane to remove insoluble matter, filter and evaporate to dryness to obtain dihydroartemisinin inclusion compound; image 3 Visible, in D 2 The peak of dihydroartemisinin appeared at Oδ0-1ppm, which proved that dihydroartemisinin had been encapsulated into the cavity by ring-opening cucurbitacin 1.
[0040] (2) Preparation of inclusion compound of dihydroartemisinin and secocucurbituril 2
[0041] Weigh 0.1868g (0.1mmol) of ring-...
Embodiment 3
[0042] Example 3: Preparation of clathrates of artemisinin and two kinds of split cucurbituril
[0043] (1): Preparation of inclusion compound of artemisinin and secocucurbituril 1
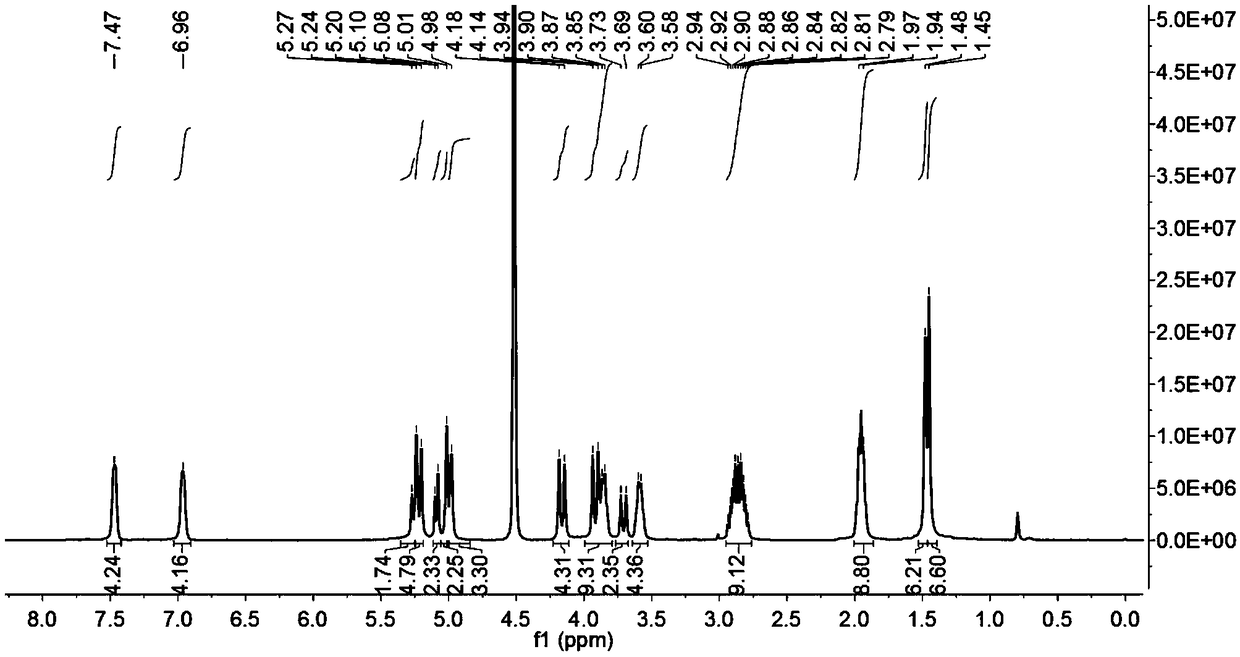
[0044] Weigh 0.1 mmol of ring-opening cucurbituril and dissolve it in 15 mL of distilled water, stir until dissolved at 30°C, weigh 0.5 mmol of artemisinin and dissolve it in 10 mL of acetone solution, mix the two solutions under stirring conditions, avoid After light stirring for 36 hours, filter with filter paper and microporous membrane to remove insoluble matter, filter and evaporate to dryness to obtain artemisinin inclusion compound; Figure 5 Visible, in D 2 The peak of artemisinin appeared at Oδ0-1.25ppm, which proved that artemisinin had been encapsulated into the cavity by split-ring cucurbitacin 1.
[0045] (2) Preparation of inclusion compound of artemisinin and secocucurbituril 2
[0046] Weigh 0.1 mmol of ring-opening cucurbituril and dissolve it in 15 mL of distilled water, stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com