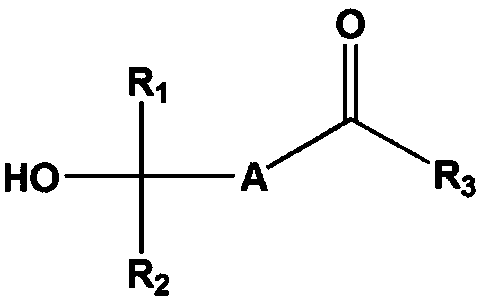
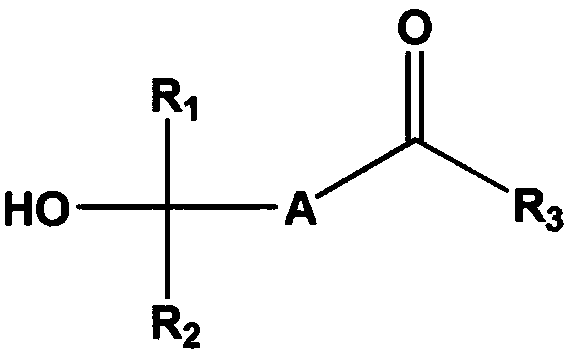
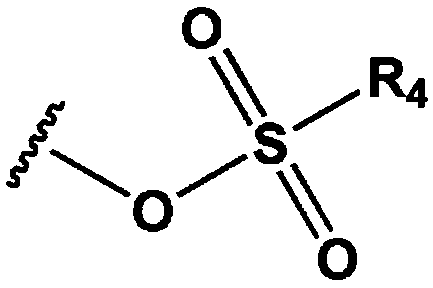
Method for producing fluorinated compounds using alcohol solvent having carbonyl group
A technology for fluorinated compounds and production methods, applied in the directions of organic chemistry methods, isotope introduction into organic compounds, esterification of saccharides, etc., can solve the problems of reduced production yield and poor production, and achieve high yield and excellent solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1: use " 3-hydroxyl-3-methyl-2-butanone " as solvent [ 18 F] Fluoropropylmethoxycarbonyltropane ([ 18 F]fluoropropylcarbomethoxytropane)([ 18 F] FP-CIT) Preparation
[0113]
[0114] to contain dissolved therein [ 18 F] An aqueous solution of fluoride (5-10 mCi) ions was passed through a cartridge (QMA) filled with ion exchange resin, and then 3.0 mL of ethanol was sprinkled on it. Dissolve 20 mg of cryptofix222-potassium methanesulfonate (K222-KOMs salt) in 1.0 mL of ethanol and splash the prepared solution on the [ 18 F] Fluoride on the cartridge, followed by elution. The cartridge was heated at 100°C while blowing nitrogen to remove ethanol. Add 4.0 mg of the starting material FP-CIT precursor ((1'R,2'S,3'S,5'S)-3'-(4-iodophenyl)-2'-(methoxycarbonyl)spiro [Azetidine-1,8'-bicyclo[3,2,1]octane]-1-ium p-toluenesulfonate) into 0.5mL 3-hydroxy-3-methyl-2-butanone , followed by reaction at 120°C for 10 minutes. Radioactive thin-layer chromatograp...
Embodiment 2
[0115] Embodiment 2: use " methyl 2-hydroxyisobutyrate " as solvent [ 18 F] fluoropropylmethoxycarbonyltropane ([ 18 F] FP-CIT) preparation
[0116] In the same manner as described in Example 1, [ 18 F] fluoropropylmethoxycarbonyltropane ([ 18 F] FP-CIT).
Embodiment 3
[0134] Embodiment 3: use " methyl 2-hydroxyisobutyrate " as solvent [ 18 F] fluoropropylmethoxycarbonyltropane ([ 18 F] FP-CIT) preparation (repeated 4 times)
[0135] This experiment was repeated four times in the same manner as described in Example 2, and the results are shown in Table 2 below.
[0136] [Table 2]
[0137]
[0138] As shown in Table 2, from the results of 5 repeated experiments using methyl 2-hydroxyisobutyrate as the reaction solvent, it was confirmed that this solvent could be used to synthesize [ 18 F] FP-CIT.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com