Method for synthesizing methanol at low temperatures
A technology for synthesizing methanol at low temperature, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc. It can solve the problems of reaction temperature limitation, achieve good stability, avoid sintering phenomenon, and be easy to prepare.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
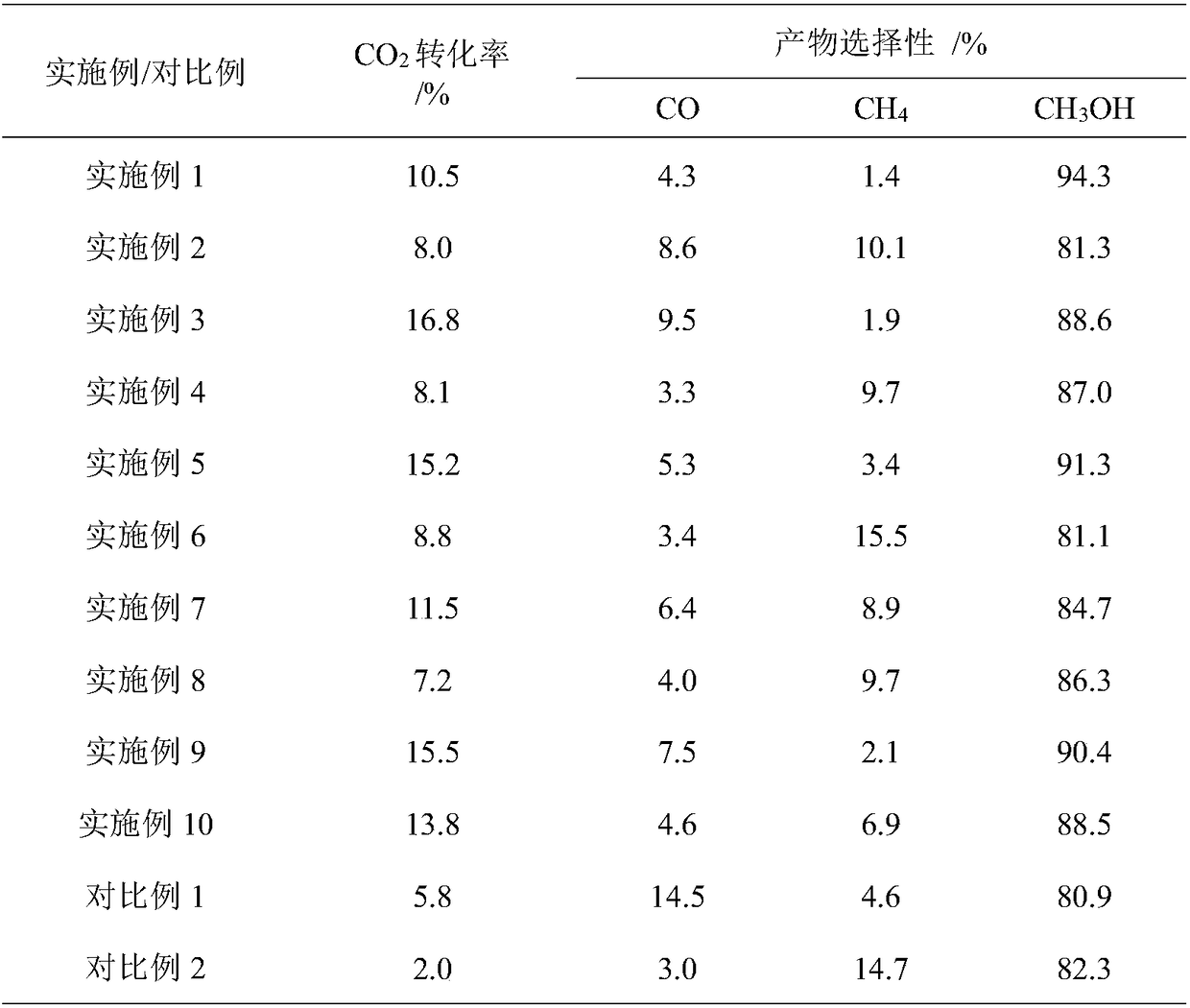
[0016] Weigh 0.5g of In with a particle size of 30-60 mesh 2 S 3 The catalyst was loaded into a fixed-bed reactor, and 10% H 2 -90% Ar mixed gas flow, at a pressure of 2bar and a space velocity of 3000mLh -1 g -1 Under certain conditions, the temperature was raised to 300°C for pretreatment of the catalyst for 1 hour, and then the temperature was lowered to 30°C. switch to CO 2 and H 2 The mixed gas atmosphere, where H 2 with CO 2 The volume ratio is 3:1, pressurized to 50bar, and the reaction space velocity is 10000mLh -1 g -1 , heated to 180°C for CO 2 Hydrogenation to methanol reaction. The reaction products were analyzed online by gas chromatography, and qualitative and quantitative analysis were performed by TCD and FID detectors, respectively. The specific reaction properties are listed in Table 1.
Embodiment 2
[0018] Weigh 0.5g of CoS catalyst with a particle size of 30-60 mesh and put it into a fixed-bed reactor, and feed 10%CO-90%N 2 Mixed air flow, at a pressure of 2bar and a space velocity of 2000mLh -1 g -1 Under certain conditions, the temperature was raised to 350°C for pretreatment of the catalyst for 1 hour, and then the temperature was lowered to 30°C. switch to CO 2 and H 2 The mixed gas atmosphere, where H 2 with CO 2 The volume ratio is 3:1, pressurized to 50bar, and the reaction space velocity is 5000mLh -1 g -1 , heated to 180°C for CO 2 Hydrogenation to methanol reaction. The reaction products were analyzed online by gas chromatography, and qualitative and quantitative analysis were performed by TCD and FID detectors, respectively. The specific reaction properties are listed in Table 1.
Embodiment 3
[0020] Weigh 0.5g of MoS with a particle size of 30-60 mesh 2 The catalyst was loaded into a fixed-bed reactor, and 10% H 2 -90%N 2 Mixed air flow, at a pressure of 1bar and a space velocity of 3000mLh -1 g -1 Under certain conditions, the temperature was raised to 300°C for pretreatment of the catalyst for 1 hour, and then the temperature was lowered to 30°C. switch to CO 2 and H 2 The mixed gas atmosphere, where H 2 with CO 2 The volume ratio is 3:1, pressurized to 50bar, and the reaction space velocity is 3000mLh -1 g -1 , heated to 180°C for CO 2 Hydrogenation to methanol reaction. The reaction products were analyzed online by gas chromatography, and qualitative and quantitative analysis were performed by TCD and FID detectors, respectively. The specific reaction properties are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com